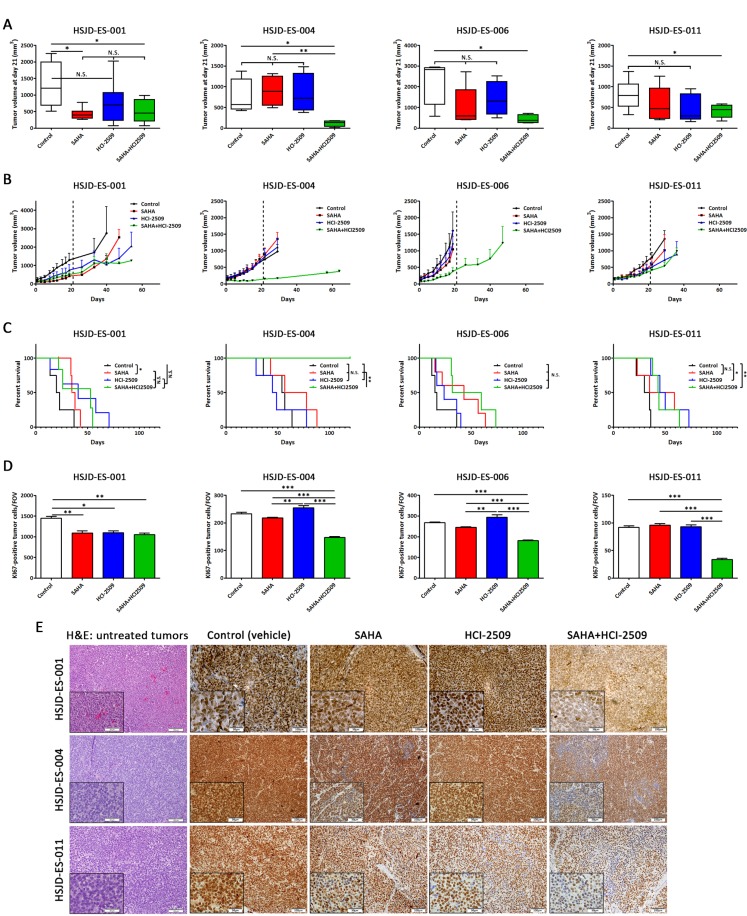
Figure 3. SAHA and HCI-2509 combination impaired tumor growth in ES PDX mice models.
(A) Tumor volumes (mm3) were measured after 21 days of SAHA, HCI-2509 and combination treatment in HSJD-ES-001, HSJD-ES-004, HSJD-ES-006, and HSJD-ES-011 PDX models. (B) Tumor growth was monitored in HSJD-ES-001, HSJD-ES-004, HSJD-ES-006, and HSJD-ES-011 PDX models upon single agent and combination therapies. Treatment was stopped after 21 days, and mice were followed until tumor volume reached 1,5 cm3. (C) Overall survival of HSJD-ES-001, HSJD-ES-004, HSJD-ES-006, and HSJD-ES-011 PDX mice treated with SAHA, HCI-2509, and its combination. Statistical tests: Log-rank (Mantel-Cox) test < 0.001 (***), 0.01 (**), and 0.05 (*). (D) Quantification of Ki67-positively labelled nuclei after 21 days of SAHA, HCI-2509, and combination treatment. Field of view (FOV). Statistical tests: significant analysis of variance, Tukey post-hoc test < 0.001 (***), 0.01 (**) and 0.05 (*). (E) Immunohistochemical staining of FLI1 in PDX tumor samples treated with SAHA and HCI-2509 alone or in combination after 21 days (20× and 40× magnifications). First column showed hematoxylin-eosin staining in untreated tumors.