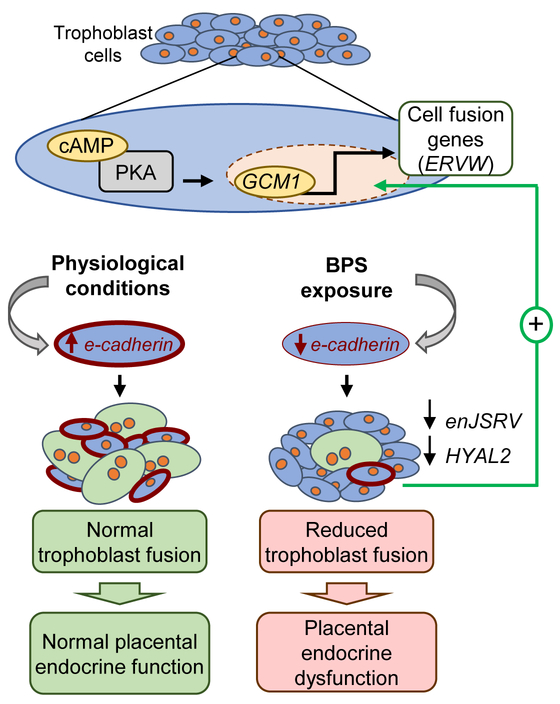
Figure 6.
BPS-induced placental dysfunction working model. Trophoblast cell fusion is a cAMP-activated of the protein kinase A (PKA) pathway which triggers the expression of the transcription factor glial cell missing factor 1 (GCM1). GCM1 in turn stimulates transcription activity of fusogenic genes, such as endogenous retrovirus envelope genes (ERVW). ERVW genes, such as enJSRV in sheep, can promote cell fusion, resulting in binucleate cell formation. However, before cell fusion can occur, cell to cell communication between two adjacent cells has to take place. E-cadherin, a transmembrane protein, facilitates cell to cell communication by the formation of adherens junctions in trophoblast cells (red cellular membranes). Under physiological conditions (left side), e-cadherin expression facilitates cell-to-cell communication enabling trophoblast fusion to occur and binucleate cells to form, resulting in normal placental endocrine function. Our findings demonstrate that gestational BPS exposure (right side) results in lower placental e-cadherin expression, preventing the formation of adherens junctions required for cell fusion and thus reducing the number of binucleate cells and expression of fusogenic genes (enJSRV and HYAL2). Altogether, this results in placental endocrine dysfunction. We hypothesize that the upregulation of GCM1 expression is a compensatory mechanism to overcome reduced trophoblast fusion rate.