Abstract
This is a single case of a 65-year-old American woman who presented with substantial weight gain and insulin resistance (IR) post-Roux-en-Y gastric bypass (RYGB) surgery. Before RYGB, she had reached 340 lbs (155 kg) and a body mass index (BMI) of 56.6 kg/m2. The surgery resulted in a 70 lbs (32 kg) weight loss, bringing her BMI, per cent total weight loss (%TWL) and per cent excess weight loss (%EWL) to 44.9 kg/m2, 20.6% and 36.8%, respectively. Unfortunately, her BMI would return to 53.6 kg/m2, nearly her pre-RYGB BMI. It was then she sought the guidance of a primary care physician with expertise in nutrition and medical management of obesity, who placed her on a ketogenic diet. One year later, she had lost 102 lbs (46.4 kg), resulting in a BMI, %TWL and %EWL of 36.6 kg/m2, 31.7%, and 63.1%, respectively, also further resulting in significant improvements of her inflammatory biomarkers. This case presentation will explore current literature, covering the effects of obesity on IR, pre-diabetes and other disease-provoking inflammatory biomarkers.
Keywords: endocrine system, diabetes, metabolic disorders, nutrition and metabolism, gastrointestinal surgery
Background
Obesity is a paramount 21st century health concern, wherein approximately 2 billion adults worldwide are either obese or overweight.1 The US Census Bureau reports that by 2050, the number of US adults aged 65 and over is expected to double, climbing from 40.2 million to 88.5 million.2 Recent studies from the Centers for Disease Control and Prevention show a prevalence of obesity among US adults aged 60 and over to be 39.8%.3 These factors combined are expected to further contribute to an already overburdened US healthcare system, increase morbidity and mortality, and drive healthcare costs to an expected 20% of US Gross Domestic Product by 2025.4
The fight against obesity in the USA has failed despite a myriad of medical interventions and educational platforms. This failure is due to a large number of complex, inter-related factors. Despite a lack of concrete evidence supporting a direct causal relationship between these, McAllister et al report two common perpetrators of the epidemic: (1) marketing practices of energy-dense/nutrient-deficient food and (2) institutionally driven declines in physical activity.5 They further report that beyond these two primarily responsible factors lay other less-proven possibilities worth considering: greater fecundity among people with higher adiposity, epigenetics, sleep debt, pharmaceutical iatrogenesis and endocrine disruptors, to name just a few.5
Weight reduction has been linked to a reduction in inflammation and inflammatory biomarkers and thus a reduction in morbidity and mortality.6 7 While diet and exercise appear lost under a looming epidemic, they remain quite effective for individual patients, particularly when it comes to lessening unfavourable inflammation profiles in patients with type 2 diabetes mellitus (T2DM).8 Moreover, when compared head to head, very-low calorie diets seem to have more favourable effects on inflammatory profiles than the Roux-en-Y gastric bypass (RYGB) in patients with T2DM.8
Within the milieu of this complex US health crisis, we explore the following case report and literature review, tracking the course of care for a 65-year-old American woman with obesity who underwent an RYGB procedure. Unfortunately, for her, the surgery subsequently failed to provide sustained weight loss. As such, she sought the care of a US licensed physician with extensive training in family medicine and nutrition for consultation and guidance through a ketogenic diet (KD) weight loss programme, which later resulted in substantial and sustained weight reduction, improvement of her symptoms and laboratory biomarkers.
Known therapeutic benefits of the RYGB and KD are discussed. Specifically, biomarkers in the context of this patient’s treatment protocol are explored: fasting insulin (FI), lipoprotein insulin resistance (LPIR), γ-glutamyl transferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), homocysteine, lipids, uric acid (UA), high sensitivity C reactive protein (hs-CRP), inflammation, glucose, haemoglobin A1c (HbA1c) and blood pressure. The authors will focus on FI and the KD. In the case of this patient, the implementation of a KD resulted in significantly reduced FI and substantial weight loss. These subsequent changes influenced the improvement of inflammatory biomarkers and eliminated her hypertension (HTN).
Case presentation
This is a single case of a 65-year-old American woman who presented with substantial weight gain post-RYGB. Before the RYGB, she steadily gained weight, reaching a maximum of 340 lbs (154.5 kg) (BMI 56.6 kg/m2), the symptoms of which were so debilitating that she was forced into medical retirement. After numerous attempts at weight loss through diet and exercise alone, she sought the care of a bariatric surgeon who performed an RYGB procedure. The surgery resulted in a 70 lbs (32 kg) weight loss, bringing her BMI, per cent total weight loss (%TWL) and per cent excess weight loss (%EWL) to 44.9 kg/m2, 20.6% and 36.8%, respectively, over the first 12 weeks of recovery. However, within a year she had regained most of the weight back, settling at 322 lbs (BMI 53.6 kg/m2) (figure 1). This was attributed to a return to her presurgical dietary habits. She had essentially experienced two failures after her RYGB: first, she had only marginally adequate initial %TWL (20.6%), and second, she had severe weight regain (74.3%).
Figure 1.
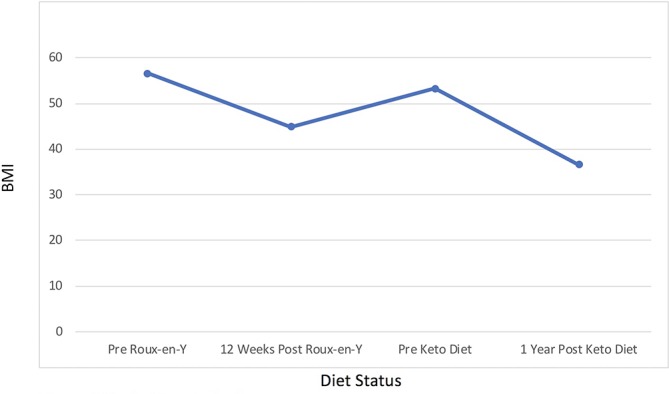
Body mass index (BMI).
This patient described her postoperative course as uncomfortable, often owing to bouts of abdominal bloating and cramping, chronic fatigue, hunger and episodes of syncope. She also suffered from a constellation of other symptoms, to include: frequent dizziness; headaches; numbness and tingling in her extremities; irritability; anxiety; allergies; sinus congestion; swollen and painful joints; knee, feet and back pain; and thin, brittle hair. She was on a plethora of medications heading into the study: omeprazole, azelastine, epinastine, cyclobenzaprine, escitalopram, lisinopril, loratadine, pindolol, pregabalin, indomethacin, clonazepam and zolpidem.
Additionally, she was taking the following supplements: melatonin, vitamin D3, riboflavin, thiamine, Centrum Silver multivitamin (for women), Caltrate 600, fish oil, ferrous sulfate and vitamin B12.
Investigations
Investigators obtained written and verbal consent, then periodically collected blood samples and measured blood pressure and weight indices over the course of 1 year. Laboratory biomarkers were assessed, which included: FI, HbA1c, homocysteine, hs-CRP, UA, total cholesterol, high-density lipoprotein (HDL), triglycerides (TGs), ferritin, glucose, LPIR, AST, ALT and GGT.
Differential diagnosis
As noted above, the patient presented with a myriad of symptoms and health concerns consistent with obesity and its many complications. The patient’s laboratory values revealed an initial inflammatory presentation. Given her increased weight and inflammatory profile, she was at increased risk for all myriad of metabolic abnormalities including pre-diabetes, metabolic syndrome (MetS), T2DM and IR.
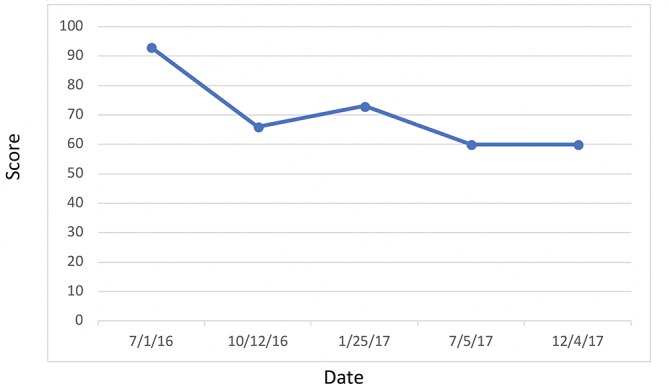
However, given her normal fasting glucose and HbA1C were normal throughout the study, diagnoses of T2DM and a pre-diabetic state were out of the question. According to the American Diabetes Association, even pre-diabetes requires an impaired fasting glucose between 100 and 125 mg/dL and has additional HbA1c based criteria of 5.7% to 6.4%.9
Nevertheless, her obesity, IR, HTN and dyslipidaemia were enough to render a diagnosis of MetS, which must include three of five diagnostic criteria, as set forth by the National Cholesterol Education Program Adult Treatment Panel III: (1) waist circumference over 40 inches (men) or 35 inches (women), (2) blood pressure over 130/85 mm Hg, (3) fasting TG level over 150 mg/dL, (4) fasting HDL cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women) and (5) fasting blood sugar over 100 mg/dL.10 Moreover, her FI and LPIR were sufficiently high on initial presentation to render a diagnosis of IR with normoglycaemia.
Treatment
Treatment goals were set, as per American Society for Metabolic and Bariatric Surgery guidelines.11 First, her ‘ideal body weight’ was defined to be the weight corresponding to a BMI of 25 kg/m2 or 150 lbs (68.2 kg) given her height of 65 inches. Second, her inflammatory biomarkers would need to improve, thus reducing her risk of insulin resistance (IR), T2DM, cardiovascular disease (CVD) and MetS.
The patient began a 1600–2400 calorie KD consisting of 70% healthy fats, coconut oil, medium chain TGs, grass-fed butter, moderate protein and very-low carbohydrates (<50 g/day). She was encouraged to eat nutrient-dense cruciferous vegetables and discouraged from eating foods high in starch. She was told to engage in a moderate, low-impact exercise programme in the form of brisk walks as tolerated. The patient received periodic dietary counselling and encouragement.
Outcome and follow-up
Within 1 year she had lost 102 lbs (46.4 kg), bringing her BMI to 36.6 kg/m2—a 20-point ΔBMI improvement from her pre-RYGB BMI (figure 1). Her %TWL and %EWL results were 31.7% and 63.1%, respectively. Many of her laboratory biomarkers improved as well as many of her symptoms. While there were no objective measures taken to establish the impact of weight loss on her quality of life, she did report discontinuation of most of her prescribed medications after her blood pressure and quality of sleep improved, anxiety abated, allergies lessened, and headaches and nerve pain subsided; indeed, she would only need to continue folic acid, escitalopram and low-dose lisinopril. And while she did gain some of the weight back after the 1 year mark (9.1 kg), her biomarkers continued to show improvements across the board, and she was quickly able to get back on track toward continued weight loss and improved overall health.
Her laboratory biomarkers are discussed here.
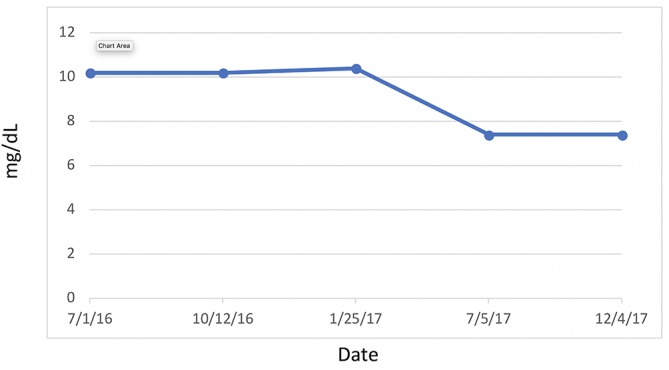
Most significantly, her FI levels dropped from 41.5 to 9.9 mIU/mL over the 1-year course of therapy (figure 2). Her weight loss had a substantial impact on many other inflammatory indices. For example, her AST, ALT (figure 3) and GGT (figure 4) all dropped to less than half their original values. Both her hs-CRP (figure 5) and UA (figure 6) levels dropped. Her LPIR had dropped 33 points, from 93 to 60 (figure 7). There were also improvements in her total cholesterol (figure 8), TGs (figure 9), low-density lipoprotein (figure 10) and HDL (figure 11), whereas the first two decreased and the later increased, respectively. Ferritin also declined nearly 150 ng/mL (figure 12).
Figure 2.
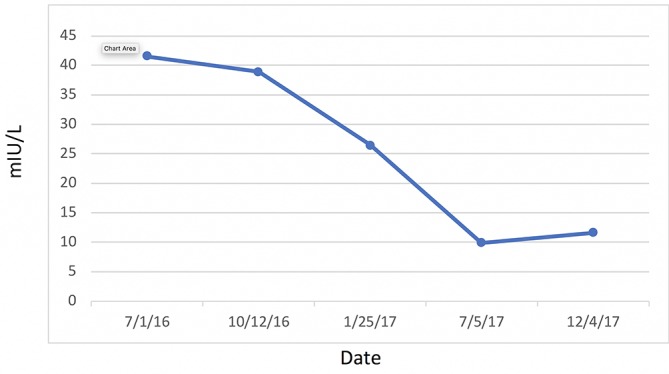
Fasting insulin.
Figure 3.
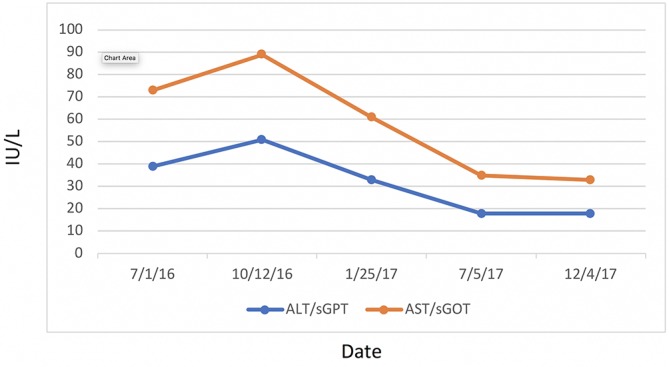
Liver function. ALT, alanine aminotransferase; AST, aspartate aminotransferase; sGOT, serum glutamic oxaloacetic transaminase; sGPT, serum glutamic pyruvic transaminase.
Figure 4.
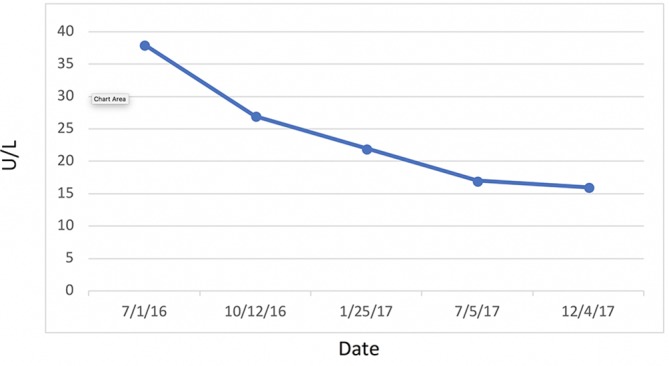
γ-Glutamyl transferase.
Figure 5.
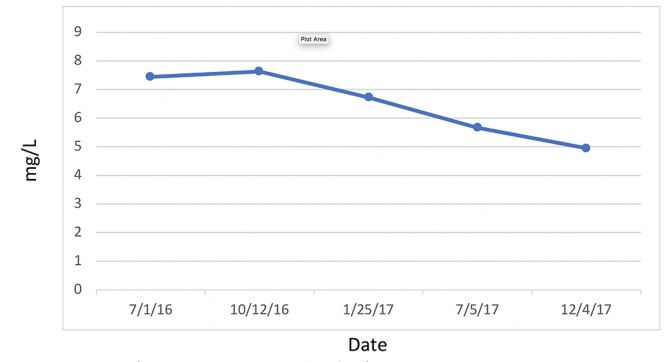
C reactive protein, cardiac.
Figure 6.
Uric acid.
Figure 7.
Lipoprotein insulin resistance.
Figure 8.
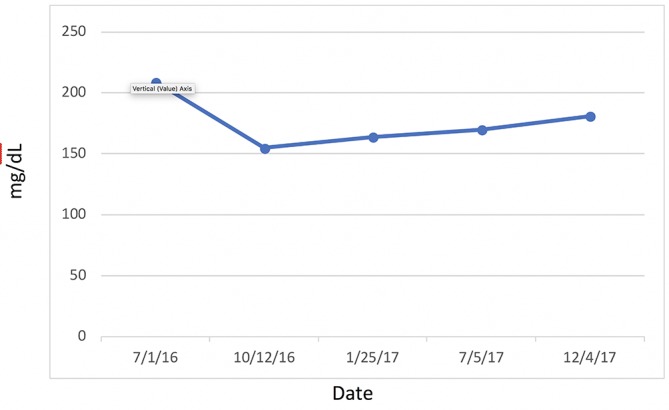
Cholesterol.
Figure 9.
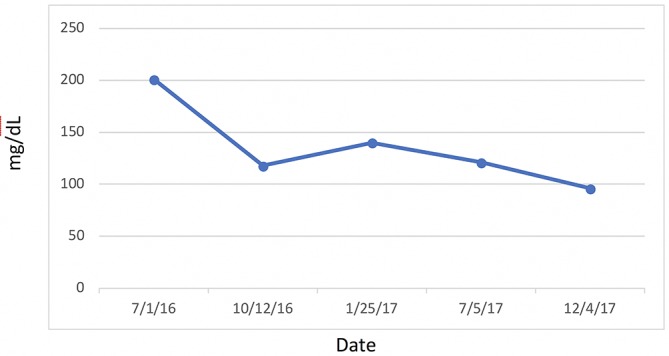
Triglycerides.
Figure 10.
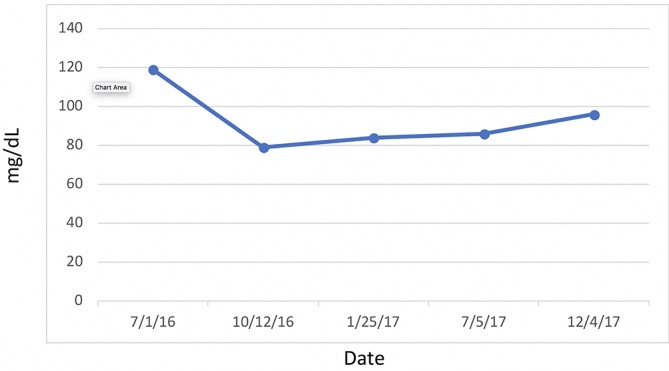
Low-density lipoprotein cholesterol.
Figure 11.
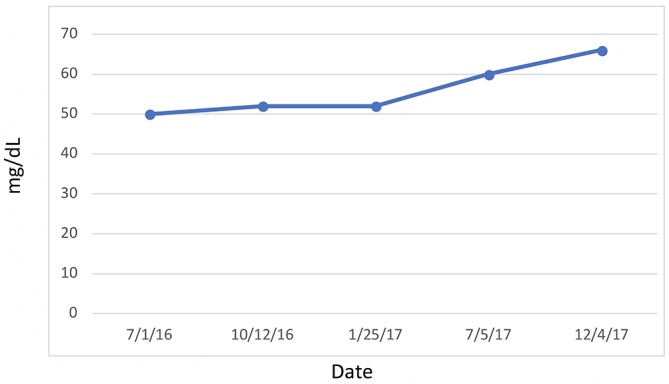
High-density cholesterol.
Figure 12.
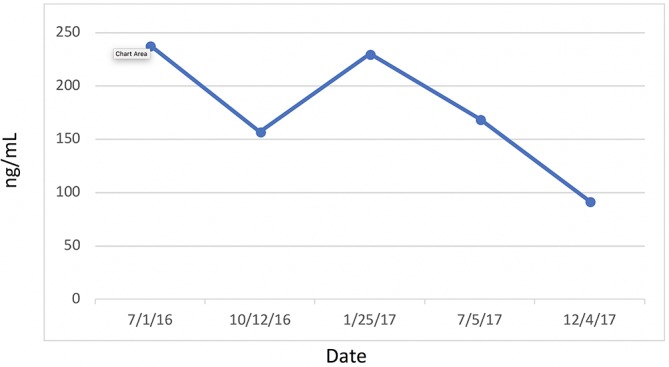
Ferritin.
Attention is directed to her HbA1C (figure 13) and fasting blood glucose (figure 14) levels—both of which were consistently within normal limits during the study. Indeed, IR, as measured by FI and LPIR, were both found to be elevated in the face of normal HbA1C and fasting blood glucose levels, thus highlighting the need for early detection of IR, rather than relying on standard HbA1C and fasting glucose indices as markers for initiating dietary therapies. Abnormal FI and LPIR scores led to the implementation of a KD for this patient. Prior to this study, given her normal HbA1C and fasting glucose levels, the patient was never informed she had a problem with carbohydrate intake overload. It was not until the discovery of her abnormal insulin indices that action was taken to correct her carbohydrate intake.
Figure 13.
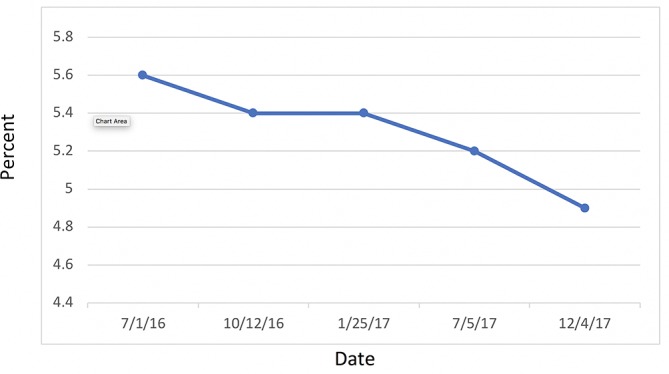
Haemoglobin A1C.
Figure 14.
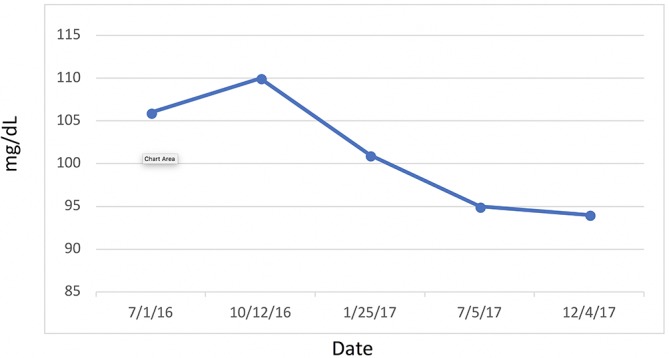
Glucose.
Discussion
Other postoperative and preoperative factors can determine success or failure of RYGB. Advanced age, higher initial BMI, preoperative weight gain and DM are predictors of weight loss failure following RYGB.12 13 Weight regain was observed in a 24-month prospective study, wherein approximately 50% of patients regained weight.14 Both surgical failure and weight regain were higher in the ‘superobese’ group.14 In a 2-year retrospective study, Ritz et al found that ‘the patients who failed, succeeded, or had intermediate results at two years post-surgery had different percentage of excess weight loss profiles during this period.’ Moreover, patients who lost less than 30% of their excess weight were unlikely to have lost greater than 50% at 24 months.15
With regard to this case, it is important to acknowledge that surgical complications have not been ruled out. Specifically, poor initial technique (large pouch size, short Roux limb, etc) or subsequent complication (pouch dilation, formation of a gastrogastric fistula, etc) could explain both her initial weight reduction failure and/or her subsequent high weight regain.
Furthermore, the authors are not proposing that diet alone is superior to bariatric surgery, for it is likely that both factors were and continued to work synergistically for this patient, yielding her significant weight loss. Before concluding that the KD is superior to any other method of weight loss, we consider the results of a 5-year follow-up analysis from the STAMPEDE trial showing that ‘bariatric surgery was superior to intensive medical therapy in terms of glycaemic control, weight reduction, medication reduction, improvement in lipid levels, and quality of life.’ 16
Physiological mechanisms and improvements in this patient’s biomarkers are further explored here.
According to Shalaurova et al, patients move through two transitional states before developing T2DM: IR with normoglycaemia followed by a state of moderate hyperglycaemia known as ‘pre-diabetes.’17 Hyperinsulinaemia is one of the first abnormalities seen in early obesity.18IR is a state of decreased cellular sensitivity to the mechanisms of insulin leading to increased pancreatic β-cell insulin secretion. Once established, IR then leads to a myriad of clinical sequelae, including but not limited to HTN, Alzheimer’s disease, CVD and T2DM.19 Therefore, early intervention in IR patients, who have yet to experience β-cell function loss and progression to pre-diabetes, prevents the development of T2DM.17
Diets high in carbohydrate, which lead to increased insulin, generate the formation of white adipose tissue (WAT) rather than brown adipose tissue (BAT).20 21 Over time, hyperinsulinaemia tends to decrease the metabolic rate, thus encouraging the storage of WAT and diminishing the formation of more metabolically active BAT.20 21 When ketones are increased through the KD, fat cells begin shifting from WAT (storage) to BAT (active).20 21BAT produces greater energy because it contains more mitochondria and is thus better able to generate heat and burn calories more efficiently. The difference between BAT and WAT is due to an insulin-induced reduction in UCP-1 (uncoupling protein 1) and PGC-1α (proliferator-activated receptor γ coactivator 1-α).20 21 This reduction possibly explains the weight gain so commonly seen in insulin-dependent T2DM.20 21
A large prospective cohort study from 18 countries found that excessive carbohydrate intake was associated with increased mortality and non-CVD mortality.22 Low-carbohydrate diets like the KD quickly diminish hepatic fat, which is known to lead to other metabolic abnormalities.23 Oxidative stress and liver inflammation, as measured by malondialdehyde, superoxide dismutase, AST, ALT and GGT increase in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).24 Basaranoglu et al reveal that ‘hepatic de novo lipogenesis (fatty acid and triglyceride synthesis) is increased in patients with NAFLD,’ further contributing to fatty liver.25
Additionally, IR and cytokine production are key mechanisms leading to NASH.26 Acyl and acetyl CoA are both precursors to cholesterol synthesis.27 Acetyl CoA is a metabolite of the breakdown of both fat and glucose. If insulin is high, it encourages acetyl CoA toward lipogenesis.28 If low, it pushes acetyl CoA toward ketogenesis. Insulin is a potent inhibitor of ketogenesis.29 Insulin also drives cholesterol production through HMG-CoA (β-hydroxy β-methylglutaryl-CoA) reductase enzyme.30 TGs are eventually converted to fat in the adipose tissue—a process facilitated via the key hormone, insulin.31 When glycogen storage capacity is maxed out within the liver, hepatocytes convert glucose to glycerol and fatty acids, which can then be used for lipogenesis. Insulin then deposits TGs into adipose tissue—the storage of which is unlimited.32
Body mass index (BMI), genetic factors, caloric intake and energy expenditure all influence obesity.1 33 MetS is a specific set of metabolic traits which increase T2DM and CVD risk over any single CVD risk factor.1 While diet and exercise have had a dramatic impact at the individual level, they have done little to improve obesity and BMI at the population level.1
WAT acts as a major secreting endocrine organ.34 Obesity encourages a number of proinflammatory mediators, such as hs-CRP, tumour necrosis factor α, interleukin 6 (IL-6) and plasminogen activator inhibitor-1.35–38 Glycaemic load is associated with increased plasma hs-CRP in IR-prone, healthy, middle-aged women.35 Proinflammatory markers are exacerbated by high consumption of rapidly digested and absorbed carbohydrates, increasing the risk of CVD.35 Low-grade systemic inflammation and C reactive protein (CRP) levels are substantially increased in obese people than they are in leaner people.36 More importantly, reduction of weight has been shown to decrease hs-CRP and thus reduce inflammation.37 38
The relationship between hs-CRP and HTN is well-established.39–45 Elevated hs-CRP is a risk factor for CVD as well as HTN, wherein HTN increases proportionate to increased hs-CRP.41 Moreover, this association is more pronounced in women who are postmenopausal, independent of BMI.39 This correlation, in theory, is due to inflammation, obesity and loss of oestrogen in postmenopausal HTN.39 CRP contributes to HTN by diminishing the production of nitric oxide (NO) from endothelial cells, resulting in vasodysregulation and endothelial dysfunction.39–42 44 The angiotensin type 1 receptor, when overstimulated, can cause inflammatory oxidative stress, vascular immune dysfunction and HTN.40 This mechanism is counterbalanced by the angiotensin type 2 receptor (AT2R).40 The endothelial NO effects of hs-CRP noted above downregulate AT2R.40 CRP activates monocytes, which in turn release proinflammatory cytokines such as interleukin 1β, IL-6, intracellular cell adhesion molecule 1, vascular cell adhesion molecule 1 and TNF-α, all of which will encourage inflammation, aldosterone and antidiuretic hormone secretion, and platelet aggregation.40 44Angiotensin receptor blockers are a group of pharmaceutical compounds designed to antagonise angiotensin 1 and 2 receptors and reduce CRP, causing vasodilation and reduction in vasopressin and aldosterone.42 43 These combined effects reduce blood pressure.42 45
Many studies have conclusively shown that GGT activity is associated with many CVD risk factors and predicts new-onset T2DM, HTN, stroke and myocardial infarction; thus, its elevation could be an indication of sustained low-grade inflammation that is associated with these conditions.46–48
The question of high plasma UA and its negative or positive association with coronary artery disease risk and MetS remains.49 Despite the fact that UA serves as 50% of the body’s endogenous antioxidant, it is still unknown whether it is a causal or protective antioxidative response.50 There is some evidence to suggest that the quantity and the duration of the concentration of the UA are what counts. Acute elevation appears to be a protective factor, whereas chronic elevation is an oxidative risk factor.49 Notwithstanding these considerations, recent studies point to consumption of high fructose corn syrup as a major factor in the elevation of UA levels and systolic blood pressure, which can ultimately lead to adverse health outcomes if consumed over enough time.51 52
Her biomarkers fluctuated under the influence of many complex physiological mechanisms; the specifics of such have been debated in the literature. Bikman et al20 21 suggest there are two camps of thought concerning weight change. The ‘calorie camp,’ currently considered conventional theory, adheres to a ‘calories in, calories out’ model, wherein excess calories determine fat gain. In essence, either calories are used for energy or they are stored. Camp 2, the ‘endocrine camp,’ supports insulin access determines fat gain. In other words, when insulin is elevated over normal baseline homeostatic levels, it will signal the adipocytes to expand the influx of energy and reduce the efflux of energy.20 21 In most cases, there is an overlap of both of these factors at work. The nature of our adipose tissue explains this variance, as adipose tissue can act differently in different environments.20 21 The amount of each and which of these takes primary precedence in determining weight gain or loss continues to be debated. However, Bikman’s research points more toward the ‘endocrine camp’ as a more accurate working theory.
Ludwig and Ebbeling’s53 recent Harvard affiliated study, which also focuses on the carbohydrate-insulin model (CIM), has further challenged the ‘calories in, calories out’ theory. According to these authors, the conventional model conflicts with recent research on the physiologic mechanisms at work in body weight determination.53 Moreover, CIM provides a more comprehensive and practical paradigm as explanation for the major influences of fat accumulation and metabolic dysfunction.53 For example, micronutrients, environmental endocrine-disrupting chemicals, protein amount and type, sleep, fatty acid profile, stress and physical activity can all directly influence insulin secretion and thus adipocyte physiology.53
The authors of this report subscribe to Bikman, Ludwig and Ebbeling’s work, wherein they believe this patient’s success was more predicated on a shifting away from a primary carbohydrate diet and moving toward a KD high in healthy fats and very-low carbohydrate foods, for example, cruciferous vegetables (< 50 g/day), thus creating a more effective calorie burning environment reliant on ketones rather than glucose. Further proof of this belief rests on the fact that this patient was not instructed to reduce caloric intake or engage in any form of intermittent fasting.
The postoperative management of RYGB mandates an interdisciplinary team, which must include the patient’s surgeon, primary care provider, endocrinologist and dietician. It is imperative that primary care providers have the proper training and tools required to manage these complex patients, who often present with chronic metabolic conditions in addition to their immediate postoperative medical and nutritional needs. Solid teamwork is key in early identification of patients at risk for poor outcomes (initial weight reduction failure or long-term excessive weight regain), which would help achieve better health in the long term.
In the case of this patient, it would appear that dietary choices and habits of consuming calorie-rich, nutrient-deficient foods lead to increased levels of insulin, which ultimately lead to excessive weight regain following RYGB. Implementation of a KD resulted in a significant decrease of FI, leading to substantial weight loss. It is possible she may have achieved even greater weight loss had she employed caloric restriction and/or intermittent fasting. However, a discussion of these weight loss strategies stretches far beyond the scope of this report, but certainly are worth further exploration.
Patient’s perspective.
Weight loss was my intent for many years, but until the focal point became health, the struggle was unattainable. Many attempts at weight loss, including bariatric surgery (Roux-en-Y gastric bypass (RYGB)), were attempted. An inundation of health issues followed including vitamin deficiencies, 17 identified medical conditions and 14 medical prescriptions. Over a period of 8 years, these accumulating results left me confronting low levels of energy, immobility, desperation and increased anxiety. Walking became a daily challenge while I concurrently experienced insatiable hunger, which seriously impeded my life.
My experience with the Keto lifestyle began with a quest for knowledge and a doctor who educated rather than merely prescribe. Given my history, I did not approach the experience with much hope, but I was rewarded quickly by stopping the compelling hunger. With both internal and external patience with the process, I began to benefit from the journey. There were no inherent difficulties following the Keto menu having had the RYGB procedure. I didn’t want to measure success by daily weighing but instead weighing only monthly, while noticing a daily improvement in how I felt. There was a strong and steady weight loss which seemed purely like an extra benefit to a focus on health. My already achieved weight loss has gone well beyond bariatric surgery. Likewise, I have reduced my number of prescriptions to three, one being folic acid, and have enjoyed the disappearance of many of my medical conditions. Mobility is a constant measure for me regarding how I am doing. It is affected if a deviation occurs. From someone who found mobility almost non-existent, I am now able to bike, swim and participate in some strength building. The amount of joy that comes with that is miraculous to me.
The journey continues. I focus on health, not weight loss; although it is a genuine and valued benefit. Life challenges remain, and opportunities to deviate from healthy eating can trigger unhealthy habits. Life trials, as an anxious person, can trip my thinking but I have found mindfulness and reflection to be helpful with my mindset. Environmentally, it has been critical for me to remove all daily temptations from home and have a family life that values that way of eating. Now, I not only have a choice but a plan for living. I would recommend the Keto plan for anyone wanting improved health and a reasonable weight.
In retrospect, I wouldn’t choose bariatric surgery nor recommend it, as it was not only less than successful but added health complications. Likewise, I do believe that my physicians were always well intended following prescribed medical practice. I am merely sorry that education, opportunity and belief in patients aren’t foremost in treatment. Having had a doctor who educated as well as advised has been fortunate. With the Keto supported life, health and all that it brings has been a blessing.
Learning points.
Early intervention of a ketogenic diet (KD) for patients with hyperinsulinaemia may result in substantial weight loss and reduction of inflammatory markers, thus reducing the risk of coronary artery disease, type 2 diabetes mellitus (T2DM), hypertension and a host of other adverse outcomes.
Clinicians should consider using fasting insulin as a biomarker in the detection of early insulin resistance and T2DM.
We recommend that future, prospective studies should investigate the short-term and long-term benefits of KD/very-low carbohydrate diets in patients undergoing bariatric surgery. Subsequently, we expect that the data will support that the odds of successful weight loss after Roux-en-Y gastric bypass are increased, if combined with a KD/very-low carbohydrate diet.
Acknowledgments
The authors thank Drs Chris Towery, Vinicius Francio, Travis Allen and Shawn Wells for their review and encouragement during the writing of this work.
Footnotes
Contributors: This patient was treated between July 2016 and December 2017 by REB. REB provided all the necessary data points, patient history and background knowledge of the case required for RTH to write the manuscript in its entirety. The scope and direction for this paper was developed and directed by REB, who also analyzed and interpreted the data. RTH conducted a full literature review. Each subsequent draft was then reviewed by REB, TLB and AAA. They then provided RTH with editorial comments and direction, which further guided him in his writing. Specifically, AAA made suggestions on an appropriate title, which they ultimately used. REB provided RTH with a handful of key research papers and discussed with him the specific physiological mechanisms he wished to focus on. TLB Suggested/guided major revision of manuscript topic, suggested major rearrangement of manuscript sections, and suggested graph axis labels, and supplied additional reference manuscripts. It was REB’s idea to focus on hyperinsulinemia and its systemic effects as the key marker to the metabolic derangements that improved with KD.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome. Eur J Pharmacol 2015;763:64–74. 10.1016/j.ejphar.2015.03.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare and Medicaid Services. Services, centers for medicare & medicaid. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2017-Press-releases-items (accessed 7 Apr 2018).
- 3.Hales CM, Carroll MD, Fryar CD, et al. . Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 2017;288:1–8. [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/Proj2015.pdf (accessed 6 Jul 2018).
- 5.McAllister EJ, Dhurandhar NV, Keith SW, et al. . Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009;49:868–913. 10.1080/10408390903372599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coimbra S, Ferreira C, Belo L, et al. . Impact of weight loss on inflammation and red blood cell biomarkers after laparoscopic gastric banding surgery. J Investig Med 2018;66:304–8. 10.1136/jim-2017-000528 [DOI] [PubMed] [Google Scholar]
- 7.Beavers KM, Nicklas BJ. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front Biosci 2011;3:168–77. 10.2741/s142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lips MA, van Klinken JB, Pijl H, et al. . Weight loss induced by very low calorie diet is associated with a more beneficial systemic inflammatory profile than by Roux-en-Y gastric bypass. Metabolism 2016;65:1614–20. 10.1016/j.metabol.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–90. 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 10.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2(5-6):231–7. 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Society for Metabolic and Bariatric Surgery. Standardized outcomes reporting in metabolic and bariatric surgery https://asmbs.org/resources/standardized-outcomes-reporting-in-metabolic-and-bariatric-surgery (accessed 7 Jun 2018). [DOI] [PubMed]
- 12.Cazzo E, Silva FP, Pareja JC, et al. . Predictors for weight loss failure following Roux-en-Y gastric. 10.1590/S0004-28032014000400011 (accessed 7 Apr 2018). [DOI] [PubMed]
- 13.Al-Khyatt W, Ryall R, Leeder P, et al. . Predictors of inadequate weight loss after laparoscopic gastric bypass for morbid obesity. Obes Surg 2017;27:1446–52. 10.1007/s11695-016-2500-x [DOI] [PubMed] [Google Scholar]
- 14.Magro DO, Geloneze B, Delfini R, et al. . Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg 2008;18:648–51. 10.1007/s11695-007-9265-1 [DOI] [PubMed] [Google Scholar]
- 15.Ritz P, Caiazzo R, Becouarn G, et al. . Early prediction of failure to lose weight after obesity surgery. Surg Obes Relat Dis 2013;9:118–21. 10.1016/j.soard.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 16.Schauer PR, Bhatt DL, Kirwan JP, et al. . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalaurova I, Connelly MA, Garvey WT, et al. . Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord 2014;12:422–9. 10.1089/met.2014.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheen AJ, Letiexhe MR, Lefèbvre PJ. Effects of metformin in obese patients with impaired glucose tolerance. Diabetes Metab Rev 1995;11(S1):S69–80. 10.1002/dmr.5610110511 [DOI] [PubMed] [Google Scholar]
- 19.Einhorn D, Reaven GM, Cobin RH, et al. . American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract 2003;9:237–52. [PubMed] [Google Scholar]
- 20.Bikman B. Benjamin bikman - “insulin vs ketones - the battle for brown fat”. https://www.youtube.com/watch?v=8t1JN0RgvO4 (accessed 7 Apr 2018).
- 21.Dallon BW, Parker BA, Hodson AE, et al. . Insulin selectively reduces mitochondrial uncoupling in brown adipose tissue in mice. Biochem J 2018;475:561–9. 10.1042/BCJ20170736 [DOI] [PubMed] [Google Scholar]
- 22.Dehghan M, Mente A, Zhang X, et al. . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–62. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 23.Yki-Järvinen H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015;7:9127–38. 10.3390/nu7115454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köroğlu E, Canbakan B, Atay K, et al. . Role of oxidative stress and insulin resistance in disease severity of non-alcoholic fatty liver disease. Turk J Gastroenterol 2016;27:361–6. 10.5152/tjg.2016.16106 [DOI] [PubMed] [Google Scholar]
- 25.Basaranoglu M, Basaranoglu G, Sabuncu T, et al. . Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 2013;19:1166–72. 10.3748/wjg.v19.i8.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irie M, Sohda T, Anan A, et al. . Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol 2016;6:13–18. 10.5005/jp-journals-10018-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agranoff BW, Benjamins JA, Hajra AK, et al. . Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edn Pennsylvania: Lippincott-Raven, 1999. [Google Scholar]
- 28.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2001;2:282–6. 10.1093/embo-reports/kve071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti KG, Johnston DG, Gill A, et al. . Hormonal regulation of ketone-body metabolism in man. Biochem Soc Symp 1978;43:163–82. [PubMed] [Google Scholar]
- 30.Harris IR, Höppner H, Siefken W, et al. . Regulation of HMG-CoA synthase and HMG-CoA reductase by insulin and epidermal growth factor in HaCaT keratinocytes. J Invest Dermatol 2000;114:83–7. 10.1046/j.1523-1747.2000.00822.x [DOI] [PubMed] [Google Scholar]
- 31.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–81. 10.1172/JCI10842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tortora GJ, Derrickson RR. Principles of Anatomy and Physiology. 12th edn Hoboken: NJ:John Wiley & Sons, 2009:980. [Google Scholar]
- 33.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997;27:325–51. 10.1023/A:1025635913927 [DOI] [PubMed] [Google Scholar]
- 34.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001;60:329–39. 10.1079/PNS200194 [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Manson JE, Buring JE, et al. . Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr 2002;75:492–8. 10.1093/ajcn/75.3.492 [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Bouter LM, McQuillan GM, et al. . Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–5. 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 37.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med 2007;167:31–9. 10.1001/archinte.167.1.31 [DOI] [PubMed] [Google Scholar]
- 38.Tchernof A, Nolan A, Sites CK, et al. . Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation 2002;105:564–9. 10.1161/hc0502.103331 [DOI] [PubMed] [Google Scholar]
- 39.Ebong IA, Schreiner P, Lewis CE, et al. . The association between high-sensitivity C-reactive protein and hypertension in women of the CARDIA study. Menopause 2016;23:662–8. 10.1097/GME.0000000000000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander W. Hypertension: is it time to replace drugs with nutrition and nutraceuticals? P T 2014;39:291–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Vongpatanasin W, Thomas GD, Schwartz R, et al. . C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation 2007;115:1020–8. 10.1161/CIRCULATIONAHA.106.664854 [DOI] [PubMed] [Google Scholar]
- 42.Ghanem FA, Movahed A. Inflammation in high blood pressure: a clinician perspective. J Am Soc Hypertens 2007;1:113–9. 10.1016/j.jash.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 43.Razzouk L, Muntner P, Bansilal S, et al. . C-reactive protein predicts long-term mortality independently of low-density lipoprotein cholesterol in patients undergoing percutaneous coronary intervention. Am Heart J 2009;158:277–83. 10.1016/j.ahj.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 44.Dinh QN, Drummond GR, Sobey CG, et al. . Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014;2014:1–11. 10.1155/2014/406960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman R, L’Allier PL, Elgharib N, et al. . Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc Health Risk Manag 2006;2:221–37. 10.2147/vhrm.2006.2.3.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lippi G, Targher G, Guidi GC. Relationship between gamma-glutamyltransferase, fasting plasma glucose, and triglycerides in the general population. Clin Chem 2007;53:1866–7. 10.1373/clinchem.2007.091512 [DOI] [PubMed] [Google Scholar]
- 47.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care 1998;21:732–7. 10.2337/diacare.21.5.732 [DOI] [PubMed] [Google Scholar]
- 48.Lee DH, Ha MH, Kim JH, et al. . Gamma-glutamyltransferase and diabetes–a 4 year follow-up study. Diabetologia 2003;46:359–64. 10.1007/s00125-003-1036-5 [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr 2012;4:12–17. 10.1186/1758-5996-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baillie JK, Bates MG, Thompson AA, et al. . Endogenous urate production augments plasma antioxidant capacity in healthy lowland subjects exposed to high altitude. Chest 2007;131:1473–8. 10.1378/chest.06-2235 [DOI] [PubMed] [Google Scholar]
- 51.Nguyen S, Choi HK, Lustig RH, et al. . Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 2009;154:807–13. 10.1016/j.jpeds.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol 2011;31:410–9. 10.1016/j.semnephrol.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med 2018. 10.1001/jamainternmed.2018.2933 [Epub ahead of print 2 Jul 2018]. 10.1001/jamainternmed.2018.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]


