Abstract
Background
The definition criteria and clinical characteristics of implant-associated infection (IAI) caused by Cutibacterium (formerly Propionibacterium) spp. are poorly known. We analyzed microbiologically proven Cutibacterium orthopedic IAI in a prospective cohort.
Methods
Patients with periprosthetic joint infections (PJI) and fixation device–associated infections (FDAI) caused by Cutibacterium spp. were prospectively included. IAI was defined by significant growth of Cutibacterium spp. and presence of at least one non-microbiological criterion for infection. The McNemar’s chi-squared or binomial test was used to compare the performance of diagnostic tests.
Results
Of 121 patients with Cutibacterium IAI, 62 patients (51%) had PJI and 59 (49%) had FDAI. 109 infections (90%) were caused by C. acnes and 12 (10%) by C. avidum. The median time from implantation until diagnosis of infection was 15.7 months (interquartile range, 5–46.5 months). Clinical local signs were present in 30 patients (28%) and radiological implant loosening in 64 patients (63%). Culture sensitivity of sonication fluid was 84%, of peri-implant tissue 84% and of synovial or peri-implant fluid 56% after 14 days of incubation.
Conclusion
Cutibacterium IAI was diagnosed late in the disease course and presented with subtle signs. Prolonged culture incubation and implant sonication improved the poor performance of conventional microbiological tests. Due to lack of reliable diagnostic tests, Cutibacterium remains difficult to detect making the diagnosis challenging.
Introduction
Bacteria belonging to the genus Propionibacterium are gram-positive, aerotolerant anaerobic rods that reside primarily in pilosebaceous follicles and are part of the normal skin microbiome. Based on species habitats, genomic topology, DNA G+C content and peptidoglycan composition, Propionibacterium acnes, P. avidum, P. granulosum and P. humerusii were recently proposed to be reclassified to the novel genus Cutibacterium [1]. In addition to the skin, Cutibacterium can be found in other parts of the body such as the oral cavity, gastrointestinal and genitourinary tract [2, 3], where they usually exist as non-pathogenic commensals. In the presence of an implant, Cutibacteria are increasingly recognized as the causative pathogen of low-grade infections affecting cardiovascular devices [4], breast implants [5], neurosurgical shunts [6], ocular implants [7], internal fracture fixation devices [8], spinal hardware [9, 10] and joint prostheses [11]. They claimed attention particularly in infections after shoulder arthroplasty [12–16], spine surgery [9, 10] and craniotomy [17].
As life expectancy is rising and novel technologies are being developed, the number of implanted devices is steadily increasing. With diagnostic techniques designed for improved diagnosis of implant-associated infections (IAI) and the use of better definition criteria for infection, Cutibacterium is increasingly recognized as true pathogen rather than contamination. These methods include prolonged incubation of microbiological specimens [2], application of novel techniques for biofilm detection, such as sonication of explanted materials [18] and implementation of molecular assays [19–21].
The reported frequency, type, treatment and outcome of IAIs caused by Cutibacterium spp. vary widely between countries, institutions and medical specialties, indicating that many challenges are unresolved, including the definition, detection and interpretation of this pathogen. Detection of Cutibacterium in culture or non-culture assays can be both, overestimated (i.e. misinterpretation of contaminant as pathogen) or underestimated (i.e. misinterpretation of pathogen as contaminant) [3, 22, 23]. Aiming at better understanding the pathogenetic role of Cutibacterium in orthopedic IAI, we conducted a prospective study to analyze the diagnostic, clinical and treatment characteristics using standardized definition criteria and an uniform diagnostic algorithm. We hypothesized, that sensitivity of diagnostic tests would be low for orthopedic IAI caused by Cutibacterium spp., rendering these infections difficult to diagnose.
Patients and methods
Study design
This prospective cohort study was conducted in a tertiary healthcare center, providing advanced specialty care to a population of 4 million inhabitants. Patient recruitment, data collection and follow-up evaluation were performed within the institutional implant infection cohort project. The study protocol was reviewed and approved by the institutional ethics committee (Ethikkommission der Charité –Universitätsmedizin Berlin) and was performed in accordance with the Declaration of Helsinki. The need for informed consent was waived.
Study population
From January 2012 through March 2018 consecutive episodes of IAI caused by Cutibacterium spp. were included and classified as periprosthetic joint infections (PJI) and fixation device–associated infections (FDAI). Each episode was evaluated by an orthopedic surgeon and infectious diseases specialist according to predefined criteria (see below). Mixed infections including high-virulent microorganism (such as Staphylococcus aureus, Escherichia coli or streptococci) were excluded, whereas co-infections with low-virulent microorganisms were included. The patients were treated according to previously published treatment recommendations [24].
Definitions
IAI was diagnosed when growth of Cutibacterium spp. in at least two intraoperative peri-implant tissue samples or sonication fluid of the removed implant (>50 CFU/ml) [18] was documented. If it only grew in synovial fluid, at least one of the following additional criteria had to be present to confirm infection [25, 26]: (i) macroscopic purulence around the implant as determined by the surgeon, (ii) presence of a sinus tract communicating with the implant, (iii) implant on view, (iv) acute inflammation in intraoperative sampled peri-implant-tissue as described by the histopathological report [27, 28] or (v) synovial fluid with >2000 leukocytes/μl or >70% granulocytes in case of PJI. Time to infection diagnosis was defined as the interval from implantation of the prosthesis or fixation device (or last surgical revision of implant) to the diagnosis of infection. IAIs were categorized according to the time of manifestation in early (<3 months after surgery), delayed (3–24 months after surgery) and late (>24 months after surgery) infections [24].
Data and strain collection
Hospital charts were reviewed with a standardized case-report form to retrieve demographic, clinical, and laboratory data. The following data was extracted: age, sex, implant type, date of primary implantation, date of diagnosis, previous revision surgery, clinical signs and symptoms, systemic inflammatory biomarkers, microbiology (including antimicrobial susceptibility testing), histopathology, leukocyte count in synovial fluid (if available), antimicrobial and surgical therapy. The radiological images were assessed at time of infection diagnosis for signs of loosening, dislocation or heterotopic ossifications for joint prosthesis or insufficient bone consolidation, defined as either delayed union (at 4–6 months) or non-union (at >6 months) for fracture fixation devices [29]. Cutibacterium strains were collected and stored in the biobank at -80°C for further microbiological testing.
Diagnostic tests
Joint aspiration was performed by an orthopedic surgeon according to standardized aseptic technique. After skin preparation with povidone iodine-alcohol, synovial fluid was aseptically collected using a sterile 18-gauge needle. If the joint was aspirated preoperatively in the outpatient department, a small skin incision was done before introducing the syringe. If no synovial fluid was obtained, the needle was repositioned without withdrawing it through the skin; no fluid was injected into the joint cavity. in case of intraoperative sampling during revision surgery, synovial fluid was aspirated before opening the joint capsule. For determination of leukocyte count and percentage of granulocytes, one ml of synovial fluid was transferred into a vial containing ethylenediaminetetraacetic acid (EDTA). In addition, one ml of synovial fluid was inoculated into a pediatric blood culture bottle (BacTec PedsPlus/F, Beckton Dickinson and Co., Shannon, County Clare, Ireland) and incubated at 36 ± 1°C for 14 days or until a positive growth was signaled, 0.1 ml of synovial fluid was inoculated on aerobic and anaerobic sheep blood agar plates (bioMérieux, Marcy L’Etoile, France) and incubated 7 days aerobically at 37°C with 5% CO2 and 14 days anaerobically at 37°C. The. remaining fluid was inoculated in thioglycolate broth (Becton–Dickinson and Company, USA) for enrichment. In addition, peri-implant fluid and 3 to 5 periprosthetic tissue samples were collected intraoperatively from the implant-bone or cement-bone interface for microbiological and histopathological analysis, if revision surgery was performed. Tissue samples were homogenized and inoculated on aerobic and anaerobic blood agar plates and inoculated in thioglycolate broth, as described above for synovial fluid. The retrieved prosthetic components were sent for sonication and processed within 6 h of removal, as previously described [30]. In brief, after adding normal saline covering most of the implant, the sonication box was vortexed for 30 s, sonicated for 1 min at 40 kHz (BactoSonic, Bandelin electronic, Berlin, Germany) and again vortexed for 30 s. The resulting sonication fluid was plated in aliquots of 0.1 ml onto aerobic and anaerobic sheep blood agar plates and 1 ml was inoculated in thioglycolate broth. Cultures were incubated at 37°C for 14 days and inspected daily for microbial growth. Microorganisms on plates were enumerated as the number of colony-forming unit (CFU)/ml sonication fluid. The colonies of each microorganism morphology were identified by standard microbiological methods using automated system VITEK 2 (bioMérieux, Marcy L’Etoile, France). Susceptibility testing was performed using gradient-strip test (E-test) by the hospital microbiology laboratory (Labor Berlin—Charité Vivantes GmbH).
Statistical analysis
Categorical variables were compared using the Fisher's exact test, for comparison of continuous variables the Mann-Whitney U test was applied. A two-sided p-value of < 0.05 was considered significant. For statistical analyses and graphics the software Prism (version 7.03; GraphPad, La Jolla, CA, USA) was used.
Results
Patient characteristics
Fourty-eight patients were excluded because of non-significant growth of Cutibacterium spp. (i.e. single tissue positive or < 50 CFU/ml in sonication) and 10 patients were excluded because of co-infection with a highly virulent pathogen. Of the remaining 121 patients with orthopedic IAI caused by Cutibacterium spp., 62 patients (51%) had PJI (including 30 hip, 19 shoulder, 12 knee and one elbow prosthesis) and 59 patients (49%) had FDAI (affecting 27 spinal hardware devices, 20 plates, 5 anchorages after rotator cuff reparation, 4 intramedullary nails, 2 fixation devices for cruciate ligament graft and one dynamic hip screw) (Table 1). Patients with PJI were older than those with FDAI (71 years vs. 55 years, p < 0.001) and the infection more often involved the lower extremity (68% vs. 27%, p < 0.001). Revision surgery at the index implant was performed in 35 patients (29%), more commonly reported in PJI than in FDAI (38% vs. 20%, p = 0.044).
Table 1. Characteristics of 121 patients with orthopedic implant-associated infections, including 62 with periprosthetic joint infections (PJI) and 59 with fixation device-associated infection (FDAI).
| Characteristic | All patients (n = 121) |
Patients with PJIa (n = 62) |
Patients with FDAIb (n = 59) |
P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median age in years | 66 (IQR, 52–75) | 71 (IQR, 62–76) | 55 (IQR, 47–71) | < 0.001* | |||||
| Sex, male | 82 (68%) | 39 (63%) | 43 (73%) | 0.330# | |||||
| Anatomic location of implant | |||||||||
| Lower extremity | 58 (48%) | 42 (68%) | 16 (27%) | < 0.001# | |||||
| Upper extremity | 36 (30%) | 20 (32%) | 16 (27%) | 0.557# | |||||
| Spine | 27 (22%) | - | 27 (46%) | - | |||||
| No. previous revisions on index implant | |||||||||
| None | 84/119 (71%) | 37/60 (62%) | 47 (80%) | - | |||||
| ≥1 interventions | 35/119 (29%) | 23/60 (38%) | 12 (20%) | 0.044# |
NOTE. Data are no. (%) of patients, if not indicated otherwise. P values were calculated between the PJI group and the FDAI group using Mann-Whitney U test (*) or Fisher’s exact test (#). IQR, interquartile range.
a Including 30 hip, 19 shoulder, 12 knee and one elbow prosthesis.
b Including 27 spinal hardware devices, 25 fracture-fixation devices (12 humerus, 5 tibia, 4 femur, 4 clavicle), 5 anchorages after rotator cuff reparation and 2 fixation devices for cruciate ligament graft.
Infection characteristics
Clinical, laboratory and radiologic features of IAI are summarized in Table 2. The median time from implantation to onset of infection was 15.7 months (IQR, 5–46.5 months). The onset of infection in FDAI was earlier than in PJI (10.0 months vs. 33.8 months, p < 0.001). This difference was also reflected by the low proportion of early infections (7%) in the PJI group (4 of 60 patients), compared to 29% in the FDAI group (16 of 55 patients, p = 0.003). Persistent or increasing pain at joint site was the most frequent clinical symptom reported in 86 patients (80%), followed by local signs of inflammation in 30 patients (28%) and sinus tract in 9 patients (8%), whereas fever was documented in only one patient (1%). Median C-reactive protein and white blood cell count were in the normal range in both groups. Radiological loosening was reported in 64 patients (63%), heterotopic ossifications were described in x-ray in 16 of 53 patients (30%) with PJI.
Table 2. Infection characteristics of 121 orthopedic implant-associated infections, including 62 with periprosthetic joint infections (PJI) and 59 with fixation device-associated infection (FDAI).
| Characteristic | All patients (n = 121) |
Patients with PJI (n = 62) |
Patients with FDAI (n = 59) |
P value |
|---|---|---|---|---|
| Median time from implantation to onset of infection in months | 15.7 (IQR, 5–46.5) |
33.8 (IQR, 8.3–58.8) |
10.0 (IQR, 2–23.3) |
< 0.001* |
| Type of infection according to onset of infection after implantation | ||||
| Early (<3 months) | 20/115 (17%) | 4/60 (7%) | 16/55 (29%) | 0.003# |
| Delayed (3–24 months) | 49/115 (43%) | 22/60 (37%) | 27/55 (49%) | 0.192# |
| Late (>24 months) | 46/115 (40%) | 34/60 (57%) | 12/55 (22%) | < 0.001# |
| Clinical findings | ||||
| Persistent or increasing pain at joint site | 86/103 (80%) | 42/55 (76%) | 44/48 (92%) | 0.061# |
| Local signs of inflammationa | 30/108 (28%) | 14/55 (25%) | 16/53 (30%) | 0.669# |
| Sinus tract | 9/108 (8%) | 6/55 (11%) | 2/29 (7%) | 0.708# |
| Fever (>38°C) at admission | 1 (1%) | 0 (0%) | 1 (2%) | 0.487# |
| Radiological findings | ||||
| Migration or loosening of the implant | 64/101 (63%) | 36/53 (68%) | 28/48 (58%) | 0.409# |
| Insufficient bone consolidationb | - | - | 14/49 (29%) | |
| Heterotopic ossifications | - | 16/53 (30%) | - | |
| Laboratory findings at admission | ||||
| Median serum C-reactive protein in mg/l | 7.5 (IQR 2.4–32.2) | 10.0 (IQR 4.1–32.2) | 5.4 (IQR 1.5–34.1) | 0.070* |
| Median blood white cell count in G/l | 7.9 (IQR 6.4–9.4) | 8.2 (IQR 6.4–9.1) | 7.6 (IQR 6.6–10.5) | 0.412* |
NOTE. Data are no. (%) of patients, if not indicated otherwise. P values were calculated between the PJI group and the FDAI group using Mann-Whitney U test (*) or Fisher’s exact test (#). IQR, interquartile range.
a Including swelling, erythema, warmth at the index joint site.
b Including delayed union (between 4 and 6 months) and non-union (after >6 months).
Performance of diagnostic tests
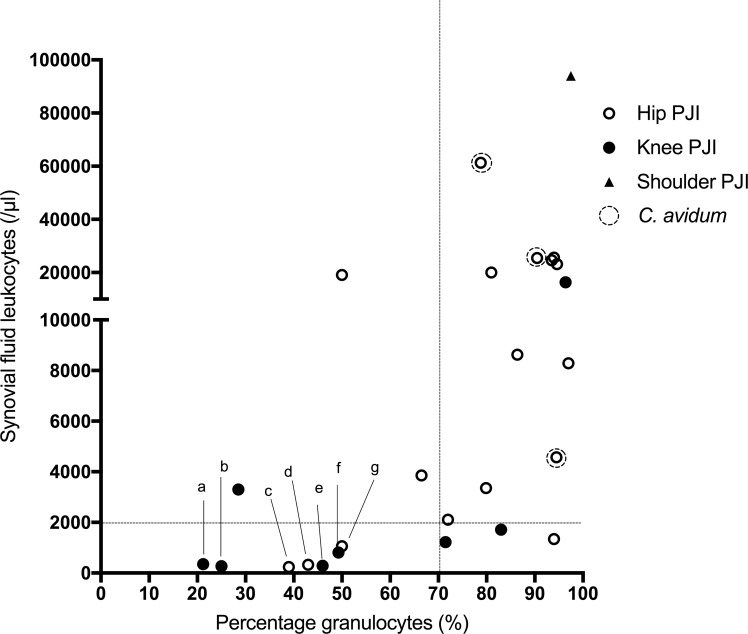
A summary of non-microbiological and microbiological tests is shown in Table 3. The C-reactive protein (CRP) was increased (>10 mg/l) in 50 of 108 patients (46%), blood white cell count was increased (>10 G/l) in 23 of 106 patients (22%). Whereas CRP was elevated in all infections caused by C. avidum, in those caused by C. acnes only 35 out of 76 patients (46%) had a value >10 mg/l (p < 0.001 (Fisher’s exact test)). There was no significant difference of median CRP between different joints in the PJI group. In patients with IAI, histopathology of peri-implant tissue showed inflammation in 47 of 74 patients (64%) with a 100% positivity rate in the subgroup of IAI caused by C. avidum. In patients with PJI, synovial fluid leukocyte count or granulocyte percentage was increased in 22 of 30 patients (73%). Fig 1 shows the distribution of the synovial fluid leukocyte count in patients with PJI affecting different joints, in whom the leukocyte count was determined. In 7 patients with microbiologically and/or histologically proven PJI, the leukocyte count was normal (dots labeled a through g in Fig 1, details showed in Table 4). Median synovial leukocyte count was higher in hip PJI cases compared to knee PJI cases (8290 leukocytes/μl vs. 1219 leukocytes/μl; p = 0.077). Only for one patient with shoulder PJI synovial fluid leukocyte count was available.
Table 3. Diagnostic tests for orthopedic implant-associated infections.
| Positive test | All patients (n = 121) |
Patients with PJI (n = 62) |
Patients with FDAI (n = 59) |
P value |
|---|---|---|---|---|
| Non-microbiological tests | ||||
| Increased serum C-reactive protein concentration (>10 mg/l) | 50/108 (46%) | 30/60 (50%) | 20/48 (42%) | 0.442# |
| Increased blood leukocyte count (>10 G/l) | 23/106 (22%) | 8/59 (14%) | 15/47 (32%) | 0.032# |
| Increased synovial fluid leukocyte count or granulocyte percentagea | - | 22/30 (73%) | - | |
| Inflammation in peri-implant tissue histopathology | 47/74 (64%) | 32/46 (70%) | 15/28 (54%) | 0.215# |
| Microbiological tests | ||||
| Body fluid cultureb | 29/52 (56%) | 20/41 (49%) | 9/11 (82%) | 0.086# |
| Peri-implant tissue culture | 87/103 (84%) | 45/61 (74%) | 42/52 (81%) | 0.502# |
| Sonication fluid culture | 79/94 (84%) | 42/52 (81%) | 37/42 (88%) | 0.404# |
NOTE. Data are no. (%) of patients. The percentages were rounded and may not sum 100%. Where the denominator is shown, percentage was calculated for the subgroup in which the test was performed. P values were calculated between the PJI group and the FDAI group using Fisher’s exact test (#).
a Defined as synovial fluid leukocyte count >2000 leukocytes/μl or percentage of granulocytes >70%.
b Synovial fluid (in case of PJI) resp. intraoperatively collected peri-implant fluid (in case of FDAI)
Fig 1. Leukocyte count and granulocytes percentage in 26 PJI patients with complete synovial fluid analysis.
The values of three patients were not depicted since the percentage of granulocytes was missing (only the leukocyte count was available). The dotted lines indicate the cutoff values for PJI definition. Seven cases with normal leukocyte count are labeled as »a« through »g« (see details in Table 4).
Table 4. PJI with negative leukocyte count in synovial fluid (see Fig 1).
CRP, C-reactive protein; PMN, polymorphonuclear cells (granulocytes); NA, not available.
| ID | Gender,Age | Joint | CRP (mg/l) | X-ray | Microbiology (positive specimen) | Pathogen | Pathology | Leukocyte count (/μl) | PMN (%) | Sinus tract | temporal appearance (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | F, 72 | Hip | 5,6 | Loosening | Sonication, tissue samples (1/2) | P. acnes | Negative | 237 | 39 | no | 30 |
| b | F, 74 | Knee | 2,5 | Loosening | Sonication | P. acnes | Negative | 273 | 25 | no | 32 |
| c | F, 73 | Knee | 0,6 | Loosening | Tissue samples (2/5) | P. acnes | Negative | 287 | 46 | no | 25 |
| d | F, 76 | Hip | 44,4 | Stable | Sonication | P. acnes | Positive | 328 | 43 | no | 98 |
| e | F, 51 | Knee | 14,62 | Loosening | Synovial fluid | P. acnes | NA | 347 | 21 | no | 11 |
| f | F, 79 | Knee | 9,3 | Loosening, ossifications | Synovial fluid | P. acnes | Positive | 813 | 49 | no | NA |
| g | M, 71 | Hip | 0,7 | Loosening | Tissue samples 2/5, sonication | P. acnes | Negative | 1059 | 50 | no | 324 |
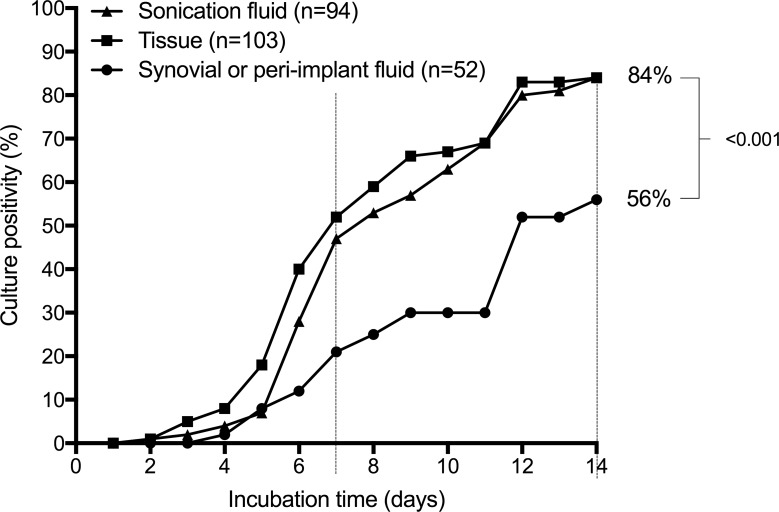
Among microbiological tests, culture of sonication fluid of the explanted orthopedic implant and of periimplant tissue showed a high detection rate of Cutibacterium (both 84%), whereas synovial or peri-implant fluid culture was significantly inferior regarding sensitivity (54%). Times to culture positivity of synovial fluid, peri-implant tissue and sonication fluid are shown in Fig 2. After 7 days of incubation the positivity rates were 21%, 53% and 47%, respectively.
Fig 2. Times to culture positivity of synovial or peri-implant fluid, peri-implant tissue and sonication fluid.
The dotted lines indicate the incubation time of 7 and 14 days.
Microbiological findings
Among 121 orthopedic IAI, 109 (90%) were caused by C. acnes and 12 (10%) by C. avidum, the latter included seven hip PJI and five FDAI involving humeral (n = 3) and femoral (n = 1) fixation plate and one anchorage in the shoulder joint. In 13 patients (11%, 10 with PJI and 3 with FDAI), co-infection with other pathogen(s) was found, including coagulase-negative staphylococci (n = 11), Granulicatella adiacens (n = 1), Finegolida magna (n = 1), Enterococcus faecalis (n = 1) and Parvimonas micra (n = 1). Two patients had a mixed infection with more than two pathogens.
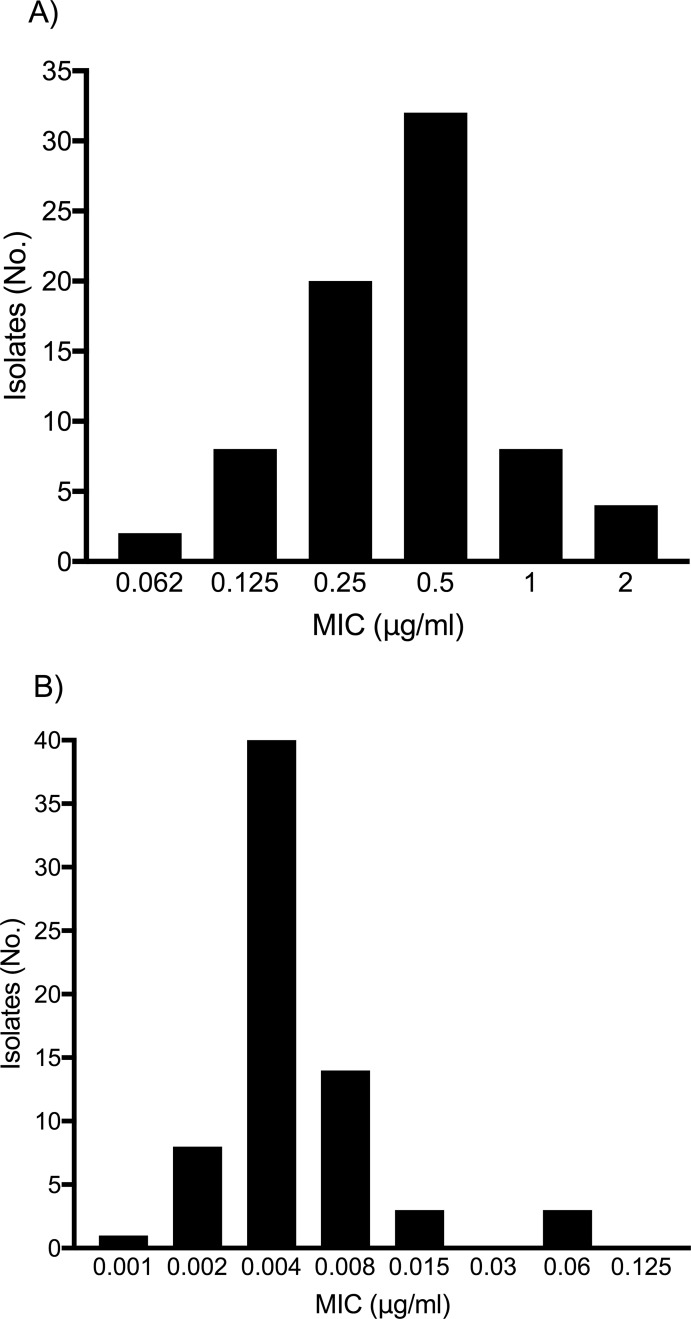
The distribution of MIC (minimal inhibitory concentration) values is presented in Fig 3. The majority of tested Cutibacterium strains had MIC values <2 μg/ml for levofloxacin (37 isolates) and MIC values <0.125 μg/ml for rifampin (32 isolates), with similar distribution in C. acnes and other Cutibacterium spp.. There are no established breakpoints for Cutibacterium spp., but authors suggested for levofloxacin low-level resistance at MIC between 0.5 μg/ml and 6 μg/ml and high-level resistance at MIC >6 μg/ml [31]. No resistance to clindamycin or amoxicillin was observed.
Fig 3.
Susceptibility of Cutibacterium spp. to levofloxacin (A) and rifampin (B), expressed as distribution of MIC values. MIC, minimal inhibitory concentration.
Surgical treatment
In PJI, two-stage exchange of the prosthesis was performed in 37 patients (60%), most commonly using a long interval of ≥6 weeks (30 of 37 patients), one-stage exchange was performed in 15 patients (24%), including six patients with only partial prosthesis exchange of the loose component due to preoperatively presumed aseptic failure. Prosthesis retention was performed in 5 patients (8%), 4 prostheses were permanently removed and one patient was treated conservatively due to impaired general condition and high surgical risk. FDAI were predominantly treated with one-stage exchange (n = 29, 49%), followed by permanent removal of the fixation device (n = 12, 25%) as sufficient bone consolidation was achieved. The remaining cases were treated with two-stage exchange (n = 10, 17%) and retention of the implant (n = 8, 14%), predominantly in acute infections presenting less than 6 weeks after implantation.
Antimicrobial treatment
The majority of patients (84 of 113 patients, 74%) was treated with a rifampin combination (combined with either amoxicillin or levofloxacin) aiming at eradicating the implant-associated infection, whereas 23 patients (20%) received a suppression treatment with amoxicillin or clindamycin and 6 patients (5%) received no antimicrobial treatment.
Discussion
Previous reports on Cutibacterium IAI predominantly described shoulder PJI [12–15]. In this cohort, we report a high proportion of infection located on lower extremities, mainly hip PJI. Similarly to other reports [8, 13, 15, 16, 32], there was a predominance of males (70% of patients in our cohort), probably reflecting the different distribution of body hair between sexes. Most Cutibacterium infections (82%) occurred late after implantation, mostly classified as delayed or late infections, as previously reported [9, 14]. Despite adequate pre-operative skin antisepsis procedures, perioperative contamination occurs as Cutibacterium spp. usually reside in the sebaceous glands located in dermal skin layers [33]. As hematogenous spread by this anaerobic pathogen is extremely rare, a long-term silent colonization of the implant before evoking inflammatory changes in the tissue must be assumed.
Slow growth, low microbial burden colonizing the implant, anaerobic growth requirement and low virulence of Cutibacterium are delaying the clinical manifestations of IAI. Importantly, persistent or increasing pain at joint site was present in the majority of patients (80%), whereas local signs of inflammation were reported only in 28%. In contrast to PJI, which mostly manifested late, approximately one third of FDAI manifested within the first three months after surgery. This difference may be explained by less soft tissue around the fixation device compared to the joint prosthesis, making local signs of inflammation earlier visible. Unspecific or subtle clinical signs and symptoms of infection evoked by Cutibacterium may suggest an aseptic etiology of the implant failure, but does not exclude a low-grade infection.
The performance of conventional preoperative and intraoperative diagnostic tests in our cohort was low, contributing to late diagnosis of IAI. In particular, laboratory parameters in serum and blood were normal in the majority of patients, as reported by others [13, 32, 34, 35]. Joint aspiration with determination of leukocyte count and microbiological analysis is the cornerstone in the preoperative evaluation of a painful prosthetic joint. However, low positivity rate of synovial fluid culture (56%) and leukocyte count (73%) was observed, as reported by others [13, 32, 35]. Interestingly, also histopathological results showed inflammation indicating infection in only 64%.
The low microbiological yield may be explained by the strong ability of Cutibacterium to adhere to the implant surface and its change from the planktonic to the biofilm phenotype. Prolonged culture incubation improved the diagnosis of Cutibacterium IAI. Only approximately one fifth (synovial or peri-implant fluid) and one half (sonication fluid) of specimens grew Cutibacterium within the first 7 days of incubation. These findings support the need for incubation period of 14 days in both aerobic and anaerobic culture media, as previously proposed [36]. Other authors highlighted an even longer incubation time of 21 days [37], however this prolongation holds the increased risk of contamination. Non-microbiological findings may support the clinical suspicion of Cutibacterium orthopedic IAI, in spite of normal laboratory and negative microbiological tests, including radiological features such as early loosening, heterotopic ossifications or insufficient bone consolidation.
Within the Cutibacterium genus, C. acnes are most common. However, other species were infrequently described, including P. avidum, P. granulosoum, P. lymphophilum and P. propionicum [2]. Data on clinical characteristics of these non-C. acnes isolates are limited to case reports. Whereas C. acnes were usually described in IAI, C. avidum caused also infections in absence of foreign bodies such as splenic or perianal abscess [38] and infections after breast surgery without implant [39–41]. Interestingly, in our cohort we found twelve IAI caused by C. avidum, of whom seven involved a hip prosthesis. In contrast to C. acnes which is commonly found in oily, sebum-rich areas, C. avidum is found only in areas rich with sweat glands, namely anterior nares, axilla, rectum and due to spread from rectum, in groin [42]. Therefore it is not surprising that majority of C. avidum infections in our cohort occurred after hip replacement, as recently shown in several reports focusing on infections after hip arthroplasty [43–45]. In line with previous reports, we noted significantly higher positivity rate of diagnostic tests for infections caused by C. avidum with 100% for elevated CRP, synovial fluid leukocyte count and periprosthetic tissue histopathology. These findings reflect the higher virulence of this specific Cutibacterium species as described in earlier reports.
Eradication of IAI is best achieved by a combination of both appropriate antimicrobial and surgical treatment. Due to its broad antimicrobial susceptibility (including to rifampin), the majority of Cutibacterium orthopedic IAI can be theoretically treated with one-stage revision, providing that the surrounding soft tissue is not compromised and all foreign material and dead tissue can be removed during debridement [24]. The activity of rifampin against C. acnes biofilms was demonstrated in vitro and in an experimental model of foreign-body infection [46, 47]. A combination regimen with rifampin was subsequently integrated in treatment recommendations. The aberrant use of antibiotics in acne may lead to the development of C. acnes strains with cross-resistance to various antibiotics with clinical impact in all diseases caused by Cutibacterium species [48]. Despite MIC values for rifampin and levofloxacin in Cutibacterium are generally in the susceptible range, emergence of resistance to both antibiotics has been reported [31, 49] and antimicrobial susceptibility testing is essential. As a dramatic decrease of clindamycin serum concentrations was shown in patients with staphylococcal osteoarticular infections treated with oral clindamycin-rifampicin combination, it is considered a second line combination partner for antibiotic regimens with rifampicin aiming at infection eradication, unless clindamycin serum concentration is thoroughly controlled [50].
In conclusion, due to heterogeneous, subtle and atypical clinical presentation, Cutibacterium IAI is often diagnosed late in the disease course. Conventional microbiological tests showed limited sensitivity, which can be improved by prolonged culture incubation and implant sonication. Due to lack of reliable diagnostic tests for low-grade IAI, some aseptic conditions may be misdiagnosed as infections and vice versa. With additional knowledge and better diagnostic tests, Cutibacterium infections are expected to be more often reliably diagnosed or excluded in future, improving the long-term treatment outcome.
Acknowledgments
Part of the results were presented at the 36th Annual Meeting of European Bone and Joint Infection Society (EBJIS) in Nantes, France, September 7–9, 2017. All authors declare no conflict of interest.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the PRO-IMPLANT Foundation (https://www.pro-implant-foundation.org) providing an educational grant to NR.
References
- 1.Scholz CF, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422–32. 10.1099/ijsem.0.001367 . [DOI] [PubMed] [Google Scholar]
- 2.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–40. 10.1128/CMR.00092-13 ; PubMed Central PMCID: PMCPMC4135900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013:804391 10.1155/2013/804391 ; PubMed Central PMCID: PMCPMC3838805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dababneh AS, Sohail MR. Cardiovascular implantable electronic device infection: a stepwise approach to diagnosis and management. Cleveland Clinic journal of medicine. 2011;78(8):529–37. Epub 2011/08/03. 10.3949/ccjm.78a.10169 . [DOI] [PubMed] [Google Scholar]
- 5.Rieger UM, Mesina J, Kalbermatten DF, Haug M, Frey HP, Pico R, et al. Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;100(6):768–74. 10.1002/bjs.9084 . [DOI] [PubMed] [Google Scholar]
- 6.Kranick SM, Vinnard C, Kolson DL. Propionibacterium acnes brain abscess appearing 10 years after neurosurgery. Archives of neurology. 2009;66(6):793–5. Epub 2009/06/10. 10.1001/archneurol.2009.75 . [DOI] [PubMed] [Google Scholar]
- 7.Deramo VA, Ting TD. Treatment of Propionibacterium acnes endophthalmitis. Current opinion in ophthalmology. 2001;12(3):225–9. Epub 2001/06/05. . [DOI] [PubMed] [Google Scholar]
- 8.Lutz MF, Berthelot P, Fresard A, Cazorla C, Carricajo A, Vautrin AC, et al. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: a retrospective study of 52 cases, 1995–2002. Eur J Clin Microbiol Infect Dis. 2005;24(11):739–44. Epub 2005/12/06. 10.1007/s10096-005-0040-8 . [DOI] [PubMed] [Google Scholar]
- 9.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2005;14(8):783–8. Epub 2005/04/21. 10.1007/s00586-004-0854-6 ; PubMed Central PMCID: PMCPMC3489259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, Yaszemski MJ, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine. 2010;35(12):1218–24. Epub 2010/05/07. 10.1097/BRS.0b013e3181c3b2f3 . [DOI] [PubMed] [Google Scholar]
- 11.Nodzo SR, Westrich GH, Henry MW, Miller AO. Clinical Analysis of Propionibacterium acnes Infection After Total Knee Arthroplasty. The Journal of arthroplasty. 2016;31(9):1986–9. 10.1016/j.arth.2016.02.025 . [DOI] [PubMed] [Google Scholar]
- 12.Hsu JE, Gorbaty JD, Whitney IJ, Matsen FA, 3rd. Single-Stage Revision Is Effective for Failed Shoulder Arthroplasty with Positive Cultures for Propionibacterium. The Journal of bone and joint surgery American volume. 2016;98(24):2047–51. Epub 2016/12/22. 10.2106/JBJS.16.00149 . [DOI] [PubMed] [Google Scholar]
- 13.Rienmuller A, Borens O. Propionibacterium prosthetic joint infection: experience from a retrospective database analysis. Eur J Orthop Surg Traumatol. 2016;26(4):429–34. 10.1007/s00590-016-1766-y ; PubMed Central PMCID: PMCPMC4856714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19(2):303–7. Epub 2009/11/04. 10.1016/j.jse.2009.07.065 . [DOI] [PubMed] [Google Scholar]
- 15.Kadler BK, Mehta SS, Funk L. Propionibacterium acnes infection after shoulder surgery. International journal of shoulder surgery. 2015;9(4):139–44. Epub 2015/12/02. 10.4103/0973-6042.167957 ; PubMed Central PMCID: PMCPMC4640005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanafani ZA, Sexton DJ, Pien BC, Varkey J, Basmania C, Kaye KS. Postoperative joint infections due to Propionibacterium species: a case-control study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49(7):1083–5. 10.1086/605577 . [DOI] [PubMed] [Google Scholar]
- 17.Chiang HY, Kamath AS, Pottinger JM, Greenlee JD, Howard MA 3rd, Cavanaugh JE, et al. Risk factors and outcomes associated with surgical site infections after craniotomy or craniectomy. Journal of neurosurgery. 2014;120(2):509–21. Epub 2013/11/12. 10.3171/2013.9.JNS13843 . [DOI] [PubMed] [Google Scholar]
- 18.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. The New England journal of medicine. 2007;357(7):654–63. 10.1056/NEJMoa061588 . [DOI] [PubMed] [Google Scholar]
- 19.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. Journal of clinical microbiology. 2010;48(4):1208–14. 10.1128/JCM.00006-10 ; PubMed Central PMCID: PMCPMC2849575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunney MM, Patrick S, Curran MD, Ramage G, Anderson N, Davis RI, et al. Detection of prosthetic joint biofilm infection using immunological and molecular techniques. Methods in enzymology. 1999;310:566–76. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 21.Street TL, Sanderson ND, Atkins BL, Brent AJ, Cole K, Foster D, et al. Molecular Diagnosis of Orthopedic-Device-Related Infection Directly from Sonication Fluid by Metagenomic Sequencing. Journal of clinical microbiology. 2017;55(8):2334–47. 10.1128/JCM.00462-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moroder P, Trampuz A, Scheibel M. Propionibacterium: We Found It, Now We Have to Deal with It: Commentary on an article by Jason E. Hsu, MD, et al.: "Single-Stage Revision Is Effective for Failed Shoulder Arthroplasty with Positive Cultures for Propionibacterium". The Journal of bone and joint surgery American volume. 2016;98(24):e112 Epub 2016/12/22. 10.2106/JBJS.16.00838 . [DOI] [PubMed] [Google Scholar]
- 23.Corvec S, Portillo ME, Pasticci BM, Borens O, Trampuz A. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012;35(10):923–34. 10.5301/ijao.5000168 . [DOI] [PubMed] [Google Scholar]
- 24.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. The New England journal of medicine. 2004;351(16):1645–54. 10.1056/NEJMra040181 . [DOI] [PubMed] [Google Scholar]
- 25.Ochsner P, Borens O, Bodler P, Broger I, Eich G, Hefti F, et al. Infections of the musculoskeletal system—Basic principles, prevention, diagnosis and treatment. 1st Edition in English ed. Grandvaux, Switzerland: swiss orthopaedics and the Swiss Society for Infectious Diseases expert group “Infections of the musculoskeletal system”; 2016. 260 p.
- 26.Renz N, Yermak K, Perka C, Trampuz A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. The Journal of bone and joint surgery American volume. 2018;100(9):742–50. 10.2106/JBJS.17.01005 . [DOI] [PubMed] [Google Scholar]
- 27.Krenn V, Morawietz L, Perino G, Kienapfel H, Ascherl R, Hassenpflug GJ, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract. 2014;210(12):779–86. 10.1016/j.prp.2014.09.017 . [DOI] [PubMed] [Google Scholar]
- 28.Ochsner PE, Hailemariam S. Histology of osteosynthesis associated bone infection. Injury. 2006;37 Suppl 2:S49–58. Epub 2006/05/03. 10.1016/j.injury.2006.04.009 . [DOI] [PubMed] [Google Scholar]
- 29.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19(4):349–56. 10.1097/01.qco.0000235161.85925.e8 . [DOI] [PubMed] [Google Scholar]
- 30.Renz N, Feihl S, Cabric S, Trampuz A. Performance of automated multiplex PCR using sonication fluid for diagnosis of periprosthetic joint infection: a prospective cohort. Infection. 2017. 10.1007/s15010-017-1073-5 . [DOI] [PubMed] [Google Scholar]
- 31.Takoudju EM, Guillouzouic A, Kambarev S, Pecorari F, Corvec S. In vitro emergence of fluoroquinolone resistance in Cutibacterium (formerly Propionibacterium) acnes and molecular characterization of mutations in the gyrA gene. Anaerobe. 2017;47:194–200. 10.1016/j.anaerobe.2017.06.005 . [DOI] [PubMed] [Google Scholar]
- 32.Figa R, Muneton D, Gomez L, Matamala A, Lung M, Cuchi E, et al. Periprosthetic joint infection by Propionibacterium acnes: Clinical differences between monomicrobial versus polymicrobial infection. Anaerobe. 2017;44:143–9. 10.1016/j.anaerobe.2017.03.008 . [DOI] [PubMed] [Google Scholar]
- 33.Heckmann N, Sivasundaram L, Heidari KS, Weber AE, Mayer EN, Omid R, et al. Propionibacterium Acnes Persists Despite Various Skin Preparation Techniques. Arthroscopy. 2018;34(6):1786–9. 10.1016/j.arthro.2018.01.019 . [DOI] [PubMed] [Google Scholar]
- 34.Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119–24. 10.1016/j.jinf.2007.02.006 . [DOI] [PubMed] [Google Scholar]
- 35.Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039–46. 10.1007/s00402-007-0454-0 . [DOI] [PubMed] [Google Scholar]
- 36.Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA 3rd, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. Journal of clinical microbiology. 2011;49(7):2490–5. 10.1128/JCM.00450-11 ; PubMed Central PMCID: PMCPMC3147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang MJ, Jancosko JJ, Mendoza V, Nottage WM. The Incidence of Propionibacterium acnes in Shoulder Arthroscopy. Arthroscopy. 2015;31(9):1702–7. Epub 2015/04/01. 10.1016/j.arthro.2015.01.029 . [DOI] [PubMed] [Google Scholar]
- 38.Wang TK, Woo PC, Yuen KY. Perianal abscess caused by Propionibacterium avidum in a cirrhotic patient. The new microbiologica. 2002;25(2):239–42. Epub 2002/05/22. . [PubMed] [Google Scholar]
- 39.di Summa PG, Yvon A, Larcher L, Raffoul W, Koch N. Propionibacterium avidum infection following breast reduction: high morbidity from a low-virulence pathogen. Journal of surgical case reports. 2015;2015(2). Epub 2015/02/13. 10.1093/jscr/rjv002 ; PubMed Central PMCID: PMCPMC4323737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kritikos A, Pagin M, Borens O, Voide C, Orasch C. Identification of Propionibacterium avidum from a breast abscess: an overlooked etiology of clinically significant infections. New Microbes New Infect. 2015;4:9–10. Epub 2015/04/02. 10.1016/j.nmni.2014.12.001 ; PubMed Central PMCID: PMCPMC4354868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panagea S, Corkill JE, Hershman MJ, Parry CM. Breast abscess caused by Propionibacterium avidum following breast reduction surgery: case report and review of the literature. J Infect. 2005;51(5):e253–5. Epub 2005/05/24. 10.1016/j.jinf.2005.04.005 . [DOI] [PubMed] [Google Scholar]
- 42.McGinley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Applied and environmental microbiology. 1978;35(1):62–6. Epub 1978/01/01. ; PubMed Central PMCID: PMCPMC242779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Achermann Y, Liu J, Zbinden R, Zingg PO, Anagnostopoulos A, Barnard E, et al. Propionibacterium avidum: A Virulent Pathogen Causing Hip Periprosthetic Joint Infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2018;66(1):54–63. Epub 2017/10/12. 10.1093/cid/cix665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wildeman P, Bruggemann H, Scholz CF, Leimbach A, Soderquist B. Propionibacterium avidum as an Etiological Agent of Prosthetic Hip Joint Infection. PLoS One. 2016;11(6):e0158164 10.1371/journal.pone.0158164 ; PubMed Central PMCID: PMCPMC4927178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeller VA, Letembet VA, Meyssonnier VA, Heym B, Ziza JM, Marmor SD. Cutibacterium (Formerly Propionibacterium) avidum: A Rare but Avid Agent of Prosthetic Hip Infection. The Journal of arthroplasty. 2018. 10.1016/j.arth.2018.02.008 . [DOI] [PubMed] [Google Scholar]
- 46.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885–91. 10.1128/AAC.05552-11 ; PubMed Central PMCID: PMCPMC3318339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayston R, Nuradeen B, Ashraf W, Freeman BJ. Antibiotics for the eradication of Propionibacterium acnes biofilms in surgical infection. J Antimicrob Chemother. 2007;60(6):1298–301. 10.1093/jac/dkm408 . [DOI] [PubMed] [Google Scholar]
- 48.Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 2017;35(2):163–7. 10.1016/j.clindermatol.2016.10.008 . [DOI] [PubMed] [Google Scholar]
- 49.Furustrand Tafin U, Aubin GG, Eich G, Trampuz A, Corvec S. Occurrence and new mutations involved in rifampicin-resistant Propionibacterium acnes strains isolated from biofilm or device-related infections. Anaerobe. 2015;34:116–9. 10.1016/j.anaerobe.2015.05.003 . [DOI] [PubMed] [Google Scholar]
- 50.Bernard A, Kermarrec G, Parize P, Caruba T, Bouvet A, Mainardi JL, et al. Dramatic reduction of clindamycin serum concentration in staphylococcal osteoarticular infection patients treated with the oral clindamycin-rifampicin combination. J Infect. 2015;71(2):200–6. 10.1016/j.jinf.2015.03.013 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.