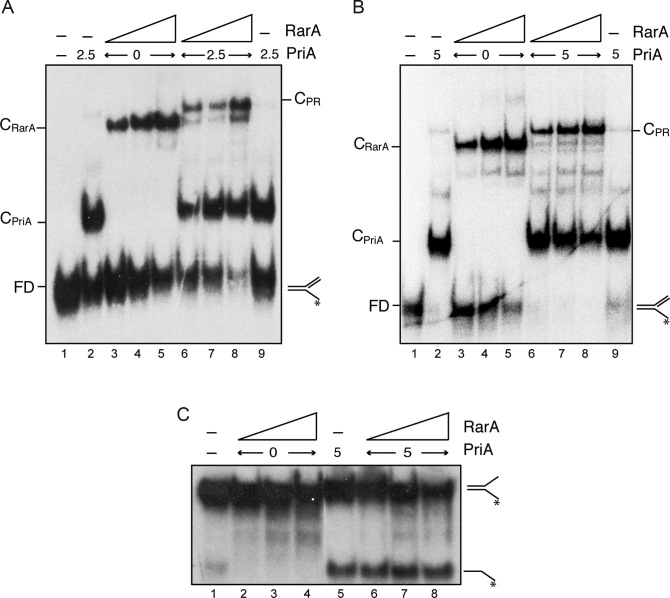
Figure 6.
RarA may interact with PriA but does not stimulate its helicase activity. (A and B) Simultaneous binding of RarA and PriA to a replicated fork. PriA (2.5 nM [A], and 5 nM [B]) and RarA (25–100 nM) were incubated with [γ-32P]-replicated fork (0.4 nM in molecules) in buffer C. Protein-DNA complexes were analysed by native PAGE and autoradiography. Abbreviations: FD, free DNA, CPriA, PriA-DNA complex; CRarA, RarA–DNA complex and CPR, PriA–DNA–RarA ternary complex. (C) RarA does not stimulate the helicase activity of PriA. The indicated combinations of PriA (5 nM) and RarA (25, 50, 100 nM) were incubated with the helicase substrate ([γ-32P]-fork) in buffer E (30 min, 30°C). Products were separated after deproteinization by PAGE and visualized by autoradiography. RarA was unable to unwind this substrate under these experimental conditions (lanes 2–4).