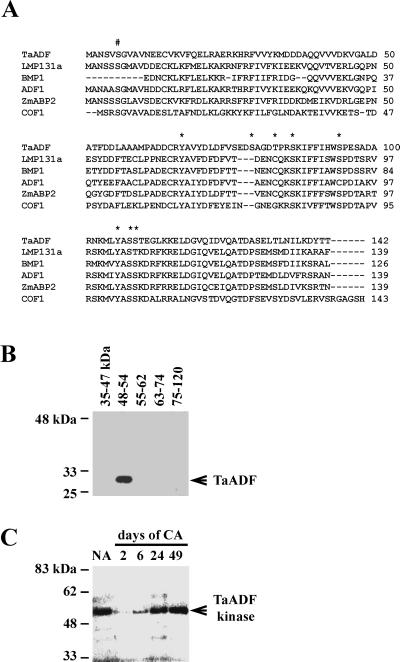
Figure 3.
TaADF acts as a substrate for a 48- to 54-kD wheat protein kinase. A, Alignment of the plant ADF/cofilins and their known/putative phosphorylation sites. LMP131a, lily ADF (Z14110); BMP1, Brassica ADF (Z14109); ADF1, Arabidopsis ADF (U48938); ZmABP2, maize actin-binding protein (X97725); and COF1, yeast cofilin (D13230). #, Conserved phosphorylation site in several eukaryotic ADFs; Asterisk, predicted phosphorylation sites in TaADF. B, SDS-PAGE was used to fractionate proteins from NA wheat (cv Norstar). After in-gel renaturation, slices of the gel corresponding to the molecular mass interval indicated above each lane were ground with purified recombinant TaADF and incubated with [γ-32P]ATP. The mixture was then loaded on a new SDS-PAGE gel and the TaADF band was revealed by autoradiography. C, In-gel phosphorylation assay of control and cold-acclimated wheat extracts. Protein extracts from wheat (cv Norstar) were separated on an SDS-PAGE gel containing 0.1 mg/mL of the recombinant TaADF. After in-gel renaturation of the proteins, the gel was incubated in kinase buffer with [γ-32P]ATP, and the band was revealed by autoradiography. 2 through 49, Duration of cold acclimation (in days).