Abstract
Primary hepatic epithelioid hemangioendothelioma (HEHE) is an extremely rare tumor of vascular origin with an incidence of <0.1 per 100 000 population. We describe a 70-year-old man with renal cell carcinoma managed via robotic right partial nephrectomy who was also found to have a solitary 10 mm lesion on a pre-operative staging computer tomography (CT) scan. The patient underwent percutaneous biopsy and local ablation of the lesion consistent with an epithelioid hepatic haemangioendothelioma. Post-procedure CT scan after 1 month showed adequate ablation margins with no new lesions. The role of local ablation for solitary, small HEHE is discussed.
INTRODUCTION
Primary hepatic epithelioid hemangioendothelioma (HEHE) is an extremely rare tumor of vascular origin with an incidence of <0.1 per 100 000 population [1]. Clinical manifestations of HEHE are heterogeneous. Initial presenting symptoms include right upper quadrant pain, and in advanced cases ascites, weight loss, anorexia and jaundice [1]. In 25% of cases, patients present asymptomatically or are an incidental finding as in our case. HEHE can present be either solitary or multi-focal whilst solitary lesion only accounts for 13–18% [1, 2]. The rarity of HEH has limited any randomized controlled trials resulting in a variety of treatment strategies. Treatment with resection or transplantation has been a matter of debate, with no studies favouring one of these strategies [3]. However, there has been no studies reporting outcomes after local ablation which maybe useful in patients with borderline fitness.
CASE REPORT
A 70-year-old gentleman with renal cell carcinoma resected by a robotic partial nephrectomy was found to have a single 10 mm lesion in segment 6 of his liver on a pre-operative staging computer tomography (CT) staging scan. Post-operative magnetic resonance imaging (MRI) confirmed low-signal intensity on pre-contrast T2-weighted images (T2-WI) which had grown slightly to s 11 mm. Following gadolinium, the lesion appeared hypovascular with no ring enhancement (Fig. 1). A contrast-enhanced ultrasound (CEUS) confirmed a hypovascular lesion in the arterial phase but had marked and progressive washout in the immediate and late phase. A PET scan showed some low grade fluorodeoxyglucose (FDG) uptake in the liver, and it was thought that the lesion may have been a primary hepatocellular carcinoma. The patient went for percutaneous biopsy and ablation of the lesion. Two core biopsies were taken and the histological appearance demonstrated a fibro-vascular lesion with numerous small, capillaries lined by atypical endothelial cells and surrounded by fibromyxoid stroma. Immunohistochemical staining had high expression of ERG, CD34, CD31 and factor XIIIa, confirming the endothelial nature and negative for Hep Par 1, CK7 and CK19. The appearances were consistent with an epithelioid haemangioendothelioma (Fig. 2). Post-procedure CT scan after 1 month showed adequate ablation margins with no new lesions.
Figure 1:
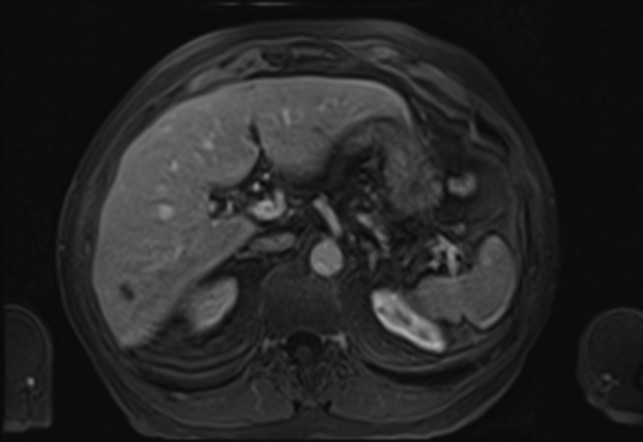
MRI Scan showing hepatic epithelioid hemangioendothelioma (HEHE).
Figure 2:
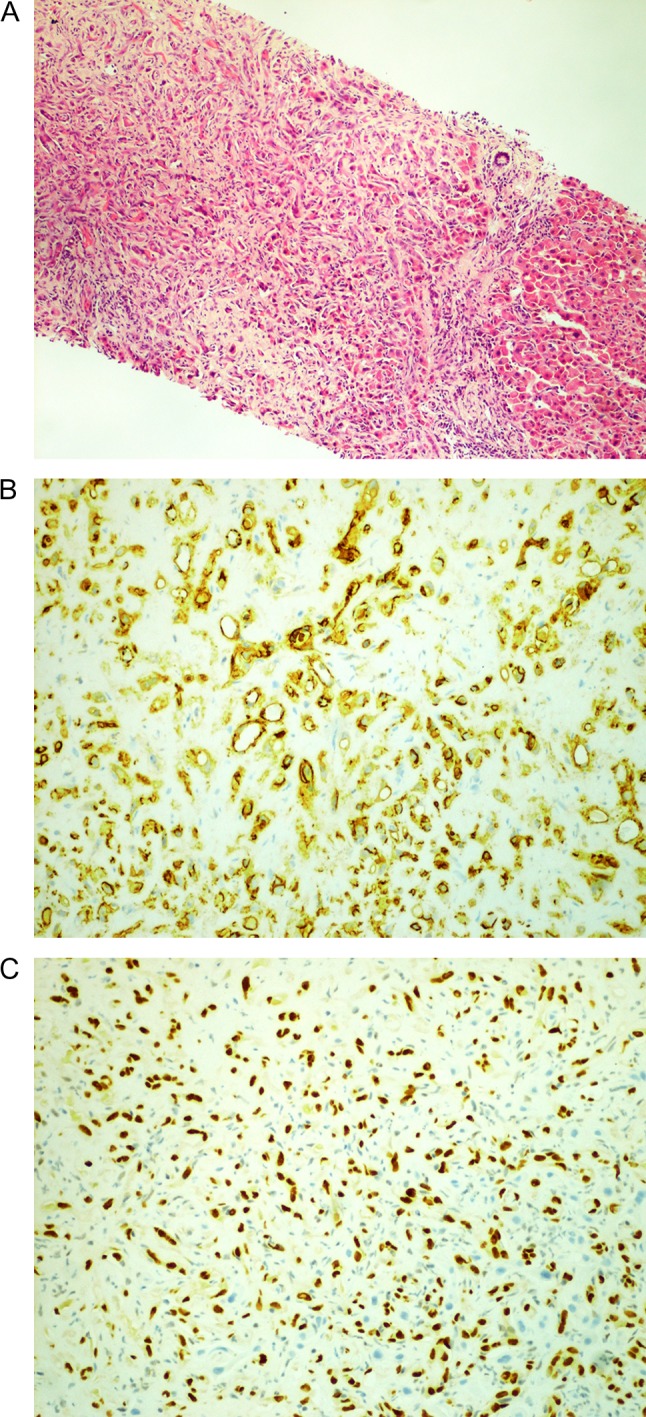
Histology images. (A) Hepatic epithelioid hemangioendothelioma (HEHE). (B) CD31 +ve and (C) ERG +ve.
DISCUSSION
To the best of our knowledge, this is the first case report to describe ablative therapy for a solitary, small HEHE demonstrating a good oncological outcome. Liver resection or transplant has been the most commonly used treatment for HEHE although numbers are small. Resection is usually preferable for solitary lesions which accounts for 10% of cases achieving a 5-year survival rate of 75%. In contrast, liver transplantation which is often the preferred therapy, given that 81% of patients have multi-focal lesions at presentation, Mehrabi et al. [1] have reported 5-year survival rate of 64% fin 110 patients undergoing OLT for HEH between 1987 and 2005. Of these, 11% died of recurrent HEHE within 5 years [4]. The literature reports an overall disease-free survival (DFS) ranging from 4 months to 10 years (mean, 59 months) [2]. Recently, Lai et al. proposed a prognostic risk score in patients undergoing liver transplantation for HEHE using the European Liver Transplant Registry to predict recurrence. This study concluded macrovascular invasion, pre-LT waiting time ≤120 days and hilar lymph node invasion were significant risk factors for recurrence [5].
RFA induces temperature changes by using high-frequency alternating current via electrodes placed within the tissue to generate areas of coagulative necrosis and tissue desiccation. A growing body of evidence has accumulated on the safety and efficacy of RFA with mortality rates between 0 and 1% and complication rates between 3 and 7% [6, 7]. RFA for hepatic neoplasm can be performed using open, laparoscopic or percutaneous approaches. Laparoscopic and open approaches increase chances of detecting unknown intra-hepatic and extrahepatic tumors as they allow complete abdominal exploration and intra-operative ultrasound assessment. Additional advantages of open and laparoscopic approaches include accurate placement of electrodes and the possible treatment of tumors in percutaneously inaccessible areas of the liver and tumors in close proximity to or invading the adjacent organs. Currently, RFA is indicated in hepatic tumors ≤3 cm as it offers similar long-term survival and recurrence rates compared to hepatic resection [8].
Owing to rarity of HEHE, choice of pre-operative imaging between CT and MRI has been a matter of debate. HEHE is classified into three types according to number of lesions: (i) solitary nodule (ii) multi-focal nodule and (iii) diffuse types. Typical imaging features, include ‘white target-like’, ‘black target-like’, ‘lollipop’, ‘strip-like’ signs, capsular contraction and submarginal distribution [9]. On non-contrast CT scan, the tumors appears as a low-density lesion with clear margin. On T1 weighted images (T1-WI), the lesions exhibit low-signal intensity whilst, on non-contrast T2-WI, the lesions exhibit heterogeneously high-signal intensity relative to the adjacent normal liver parenchyma. On diffusion weighted images (DWI), the lesions appear with slightly high-signal intensity cores and a high-signal intensity halo; appearing as ‘target-like’ configurations, present in 61% of lesions.
On contrast-enhanced MRI, the enhancement features vary according to blood supply patterns. Multi-focal lesions can be classified into four categories according to enhancement patterns: (i) slightly irregular homogeneous enhancement; (ii) peripheral enhancement with central low-signal intensity in the arterial phase, and enhanced lesions surrounded by a thin hypointense ring in the portal venous and delay phases (‘black target-like’ sign); (iii) nodular enhancement in the central part of the lesion in the arterial phase surrounded by ring-like enhancement in the portal venous and delay phases (‘white target-like’ sign); and (iv) peripheral nodular enhancement in the arterial phase and centripetal enhancement in the portal venous and delay phases, an enhancement pattern more commonly indicated in hemangioma [9]. Gan et al. [9] demonstrated that 79% of HEHE lesions exhibited a ‘target-like’ sign on T2-WI and 70% of lesions exhibited the ‘black target-like’ sign on contrast-enhancement scans. Fan et al. [10] demonstrated that HEHE possess slightly increased signal intensity with increased signal intensity centers on the T2-WI, and ‘ring-like’ peripheral enhancement on the post-contrast enhanced MRI. Bruegel et al. [11] indicated that HEH showed a ‘target-like’ sign on T2 and DWI and the lesions also showed a variable degree of peripheral rim enhancement. Collectively, these highlight that HEHE features are better defined using a variety of MRI sequences rather than CT, allowing for accurate diagnosis.
In summary, this is the first case report to describe the management of a patient with solitary, small HEHE using local ablation achieving a good oncological outcome. Future reports and studies should aim to evaluate outcomes of ablation for this indolent tumor, when feasible in comparison to liver resection.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.
REFERENCES
- 1. Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108–21. [DOI] [PubMed] [Google Scholar]
- 2. Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 1999;85:562–82. [DOI] [PubMed] [Google Scholar]
- 3. Mehrabi A, Kashfi A, Schemmer P, Sauer P, Encke J, Fonouni H, et al. Surgical treatment of primary hepatic epithelioid hemangioendothelioma. Transplantation 2005;80:S109–12. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez JA, Becker NS, O’Mahony CA, Goss JA, Aloia TA. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg 2008;12:110–6. [DOI] [PubMed] [Google Scholar]
- 5. Lai Q, Feys E, Karam V, Adam R, Klempnauer J, Oliverius M, et al. Hepatic epithelioid hemangioendothelioma and adult liver transplantation: proposal for a prognostic score based on the analysis of the ELTR-ELITA Registry. Transplantation 2017;101:555–64. [DOI] [PubMed] [Google Scholar]
- 6. Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg 2003;237:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg 2006;141:181–90. [DOI] [PubMed] [Google Scholar]
- 8. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569–77; quiz 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gan LU, Chang R, Jin H, Yang LI. Typical CT and MRI signs of hepatic epithelioid hemangioendothelioma. Oncol Lett 2016;11:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan F, Yang X, Zhu B, Zhang Y. Clinical and radiological characteristics of Chinese patients with hepatic epithelioid hemangioendothelioma. Ann Saudi Med 2013;33:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ. Hepatic epithelioid hemangioendothelioma: findings at CT and MRI including preliminary observations at diffusion-weighted echo-planar imaging. Abdom Imaging 2011;36:415–24. [DOI] [PubMed] [Google Scholar]


