Abstract
Pancreatic beta-cells respond to increasing blood glucose concentrations by secreting the hormone insulin. The dysfunction of beta-cells leads to hyperglycemia and severe, life-threatening consequences. Understanding how the beta-cells operate under physiological conditions and what genetic and environmental factors might cause their dysfunction could lead to better treatment options for diabetic patients. The ability to measure calcium levels in beta-cells serves as an important indicator of beta-cell function, as the influx of calcium ions triggers insulin release. Here we describe a protocol for monitoring the glucose-stimulated calcium influx in zebrafish beta-cells by using GCaMP6s, a genetically encoded sensor of calcium. The method allows monitoring the intracellular calcium dynamics with single-cell resolution in ex vivo mounted islets. The glucose-responsiveness of beta-cells within the same islet can be captured simultaneously under different glucose concentrations, which suggests the presence of functional heterogeneity among zebrafish beta-cells. Furthermore, the technique provides high temporal and spatial resolution, which reveals the oscillatory nature of the calcium influx upon glucose stimulation. Our approach opens the doors to use the zebrafish as a model to investigate the contribution of genetic and environmental factors to beta-cell function and dysfunction.
Keywords: Biology, Issue 137, Beta-cells, calcium, GCaMP, live-imaging, function, diabetes, zebrafish
Introduction
Our blood-glucose is maintained in a narrow range, in large part due to the function of the endocrine pancreas. The endocrine role of the pancreas is performed by the islets of Langerhans, which contain hormone-secreting cells. The insulin-secreting beta-cells are responsible for reducing the levels of blood-glucose following a carbohydrate-containing meal. Inadequate insulin secretion from beta-cell can result in diabetes1, which is characterized by sustained high blood-glucose. Type 1 and Type 2 Diabetes, which currently afflicts more than 400 million people worldwide, leads to morbidity and mortality2. By investigating the molecular and environmental factors that contribute to beta-cell dysfunction, we will understand better how type 2 diabetes starts and progresses. In addition, the ability to differentiate human stem cells into functional beta-cells in vitro can provide a source of new beta-cells for cell-replacement therapies in Type 1 Diabetes. To this end, it is important to study beta-cell function and maturation in genetic model organisms in order to gain the necessary knowledge for making functional beta-cells in a dish.
Beta-cell functionality can be monitored at the whole-islet level by quantifying the total amount of insulin secreted in response to glucose-stimulation. This cumulative approach studies the islet as a single group of cells without differentiating a cell's individual properties. However, the analysis of glucose responses of individual beta-cells revealed a diversity in the functional properties of beta-cells and the presence of heterogeneity3. To assess the function of individual beta-cells, it is possible to monitor the intracellular changes that lead to insulin secretion4. Insulin secretion is preceded by an entry of glucose into the beta-cells. The glucose that enters in the beta-cells is rapidly metabolized to ATP. Higher intracellular ATP concentrations decrease the open probability of ATP-sensitive potassium ion channels leading to beta-cell depolarization. Depolarization opens the voltage-sensitive calcium ion channels and increases intracellular calcium. In turn, calcium triggers exocytosis of insulin, which is released in the circulation and lowers glucose levels by promoting glucose-utilization5,6,7.
Several strategies have been applied to investigate the function of beta-cells, including monitoring of the membrane potential8, direct visualization of insulin-vesicles exocytosis9, and quantification of intracellular Ca2+ influx as a proxy for glucose-responsiveness10. Among them, imaging of intracellular Ca2+ provides the advantage of up-scaling the analysis to multiple individual cells within the same islet11,12, allowing for direct comparison of the glucose-responsiveness between individual beta-cells. Intracellular Ca2+ concentration can be monitored using calcium-sensitive fluorescent dyes13 or genetically-encoded calcium indicators (GECIs)14. Whereas calcium-indicator dyes lack cell-type specificity, GECIs can be expressed in a specific cell type by specific promoters. Additionally, the new generation of GECIs, such as GCaMP6, provides better signal-to-noise ratio along with faster temporal dynamics15. Here we describe the utility of the new-generation of GECIs, in particular GCaMP6s, to visualize calcium in beta-cells at single-cell resolution. We apply this method to the zebrafish primary islet as our model of choice. During embryonic development, beta-cells in the primary islet originate from the dorsal and ventral pancreatic buds16. The primary islet is located at a stereotypical anatomical position within the zebrafish pancreas, enabling its easy identification and isolation. Beta-cells in the primary islet are necessary for glucose-regulation, as their genetic ablation leads to hyperglycemia17,18. Furthermore, these beta-cells become glucose-responsive during early zebrafish development19. This protocol can be also applied to imaging the secondary islets, which form during the post-embryonic stages. The protocol allows to image beta-cells ex vivo, at later stages of development and under defined glucose concentrations.
Protocol
All the procedures including animal subjects have been approved by the Animal Welfare Act and with permission of the Landesdirektion Sachsen, Germany (AZ 24–9168.11-1/2013-14, T12/2016).
1. Preparation
NOTE: This protocol is for ex vivo imaging of zebrafish primary islet from double transgenic Tg(ins:nls-Renilla-mKO2; cryaa:CFP); Tg(ins:GCaMP6s; cryaa:mCherry)19 zebrafish. In this transgenic line, the insulin promoter (ins) drives beta-cell specific expression of two transgenes: nls-Renilla-mKO2, which marks the nucleus of beta-cells with monomeric Kusabira orange 2 (mKO2) fluorescence; and GCaMP6s15, which emits green fluorescence in response to increase in intracellular calcium levels. The beta-cell-specific expression of GCaMP6s enables studying the glucose-responsiveness of beta-cells without interference from calcium changes in surrounding cell types.
Prepare fresh fibrinogen stock (10 mg/mL) by dissolving 10 mg of bovine fibrinogen in 1 mL of Ca2+/Mg2+-containing Hanks' Balanced Salt solution (HBSS) in a 1.5 mL centrifuge tube. Vortex vigorously until the fibrinogen powder dissolves completely. Keep the solution at room temperature for at least 15 min more. NOTE: The stock can be kept at room temperature for 2–3 h. Discard the stock if the solution starts to polymerize and becomes viscous.
Prepare the fibrinogen working solution (3.3 mg/mL) by diluting the fibrinogen stock three-fold in HBSS. For example, mix 300 µL of fibrinogen stock in 600 µL of HBSS to prepare 900 µL of fibrinogen working solution.
Prepare thrombin solution (10 U/mL) by dissolving 10 units of thrombin in 1 mL of HBSS or phosphate buffered saline (PBS). NOTE: This solution can be prepared in advance, aliquoted in 50 µL parts, and frozen at -20 °C.
Prepare 200 mM D-glucose solution by dissolving 1.8 g of D-Glucose in 50 mL of water. Keep in 4 °C for long-term storage.
Prepare 300 mM KCl solution by dissolving 1.1 g of KCl in 50 mL of water. Keep in 4 °C for long-term storage.
Procure 35 mm diameter glass-bottom dishes for mounting the islets.
For dissection and mounting of the islet, use a stereo microscope equipped with fluorescence lamp and red filter cube (TRITC: excitation: 532–554 nm, emission: 570–613 nm; or Texas-Red: excitation: 540–580 nm, emission: 592–667 nm).
For imaging the calcium influx in beta-cells, use an inverted confocal microscope (Zeiss LSM 780 or similar) with 20X (0.8 NA) air objective and equipped with a plate holder for the 35 mm diameter glass-bottom dishes.
Prepare a 200 mg/L solution of tricaine methane sulfonate (MS222) for euthanizing the zebrafish.
Procure 90 mm Petri dishes for the zebrafish dissection.
2. Zebrafish Primary Islet Dissection and Mounting
Euthanize a fish using prolonged incubation in MS222. For this, gently remove the fish from the fish tank using a fishing net and incubate it in a Petri dish containing the MS222 solution until the animal shows no opercular (gills) movement; typically, this takes 5 min. Transfer the fish to a Petri dish containing the HBSS solution with Ca2+/Mg2+.
- Under a stereo microscope equipped with a fluorescence lamp and red filter cube, dissect the skin covering the right side of the abdomen of the animal to isolate the pancreas.
- For this, cut the skin of the zebrafish from mouth to anal fin using sharp forceps. Peel away the cut skin to expose the abdomen; this snipping exposes the internal organs. By using the red fluorescence of mKO2 expression in beta-cells, ascertain the location of the islets by visual examination under the microscope. If needed, remove the lobes of the liver, as they could cover the islet, making it difficult to find. NOTE: The primary islet is located near the anterior region of the abdomen, typically on the right side.
Clean the primary islet by carefully removing surrounding tissue such as liver and adipocytes. Take precaution not to injure or poke the islet; after clearing of the surrounding tissue, the individual cells on the islet surface become discernable.
Pipette a 30 µL drop of HBSS onto the center of a glass-bottom dish. Transfer the dissected islet to this drop.
Carefully wash the islet once with HBSS and once with 30 µL of the fibrinogen working solution (3.3 mg/mL). Make sure to avoid drying of the islet during the washing steps because failure to do so results in cell death.
Slowly and gently add 10 µL of the thrombin solution (10 U/mL). Leave the islet and the dish untouched for 15–20 min. Observe that the fibrinogen-thrombin drop will become viscous and slightly opaque at this point.
3. Ex Vivo Live-imaging of GCaMP Fluorescence Intensity in Zebrafish Primary Islets
Add 200 µL of HBSS on top of the mold and place the dish carefully on the plate holder of the confocal microscope. Use a 20X, 0.8 NA, air objective for confocal imaging. Locate the islet using the brightfield option.
Using the filter for red fluorescence to view the nuclear mKO2 fluorescence in beta-cells, focus on the islet. Individual nuclei should be clearly visible.
Locate a clear imaging plane by manually changing the focal plane of the confocal microscope so as to move through the thickness of the islet along its z-axis. Ensure that the imaging plane contains a sufficient number (50–100) of beta-cells for imaging, and the brightness of the nuclear mKO2 fluorescence is uniform, especially in the center of islet.
Set up a sequential acquisition for GCaMP6s and mKO2 fluorescence using the following settings in the "Smart Setup Menu": GCaMP6s, excitation: 488 nm, emission: 500–555 nm, false-color: green (Select "GFP"); mKO2, excitation: 561 nm (mCherry), emission: 570–630 nm, false-color: red (Select "mCherry"). NOTE: With this setting, the red channel will record the position of beta-cell nuclei, while the green channel will record the GCaMP fluorescence intensity.
In the "Acquisition Mode" set the image resolution at 1,024 x 1,024 pixels, speed at 10 and averaging at 1. Initiate a continuous recording by selecting the option for "Time-series", and setting the "Duration" for 500 cycles, with approximately 2 s acquisition time per frame. NOTE: The first 50 frames of the time-series correspond to the activity of the beta-cells at 5 mM glucose concentration. This is the baseline response. A responding beta-cell will show waxing and waning in the green fluorescence intensity with time. We have observed that a few (1–5%) of the beta-cells oscillate at 5 mM glucose.
- Keep an eye on the imaging cycle. After the first 50 frames, increase the glucose concentration of the surrounding solution to 10 mM without stopping the recording.
- Without perturbing the image acquisition, gently pipette 5 µL of the 200 mM D-glucose solution on top of the gel holding the islet. Acquire 150 frames at 10 mM glucose. NOTE: The increase in glucose concentration will increase the number of beta-cells undergoing GCaMP fluorescent oscillations in the green channel.
- Ensure that the nuclei of the cells remain stable during the process. If the islet is shaking extensively during acquisition and the nuclei are moving out of the focal plane, discard the sample (if needed).
- Wait a sufficient period of time to enable polymerization of the fibrinogen-thrombin mold in order to ensure the stability of subsequent samples.
At 200 frames, further increase the concentration of the solution to 20 mM by gently pipetting 10 µL of the 200 mM D-glucose solution. Acquire 150 frames for the 20 mM concentration.
After 350 frames, depolarize the islet using 30 mM of KCl. For this, add 20 µL of the 300 mM KCl stock solution. At this stage, observe that the GCaMP6s fluorescence oscillations will stop and fix at a high intensity; beta-cells that did not respond to glucose might also display an increase in green-fluorescence intensity upon KCL addition.
4. Quantification of GCaMP Fluorescence Trace for Individual Beta-cells
NOTE: To trace and quantify the responses of individual beta-cells to varying levels of glucose, quantify the GCaMP fluorescence intensity for the entire imaging period. Quantification is performed at cellular resolution. For this, use FIJI20 to extract the intensity values of GCaMP fluorescence from the images (steps 4.1–4.6), and spreadsheet software or R21 to perform analysis (steps 4.8–4.9).
Open the image file in FIJI using the "LSM Toolbox". For this, select "Plugin | LSM Toolbox | Show LSM toolbox". In the "LSM Toolbox", click "Open LSM" and select the image file. Note: For formats not supported by the "LSM Toolbox", convert them first to tiff for analysis.
Extract the responses of cells using the region-of-interest (ROI) tool in FIJI. Open the "ROI Manager" in the "Analyze" menu under "Tools". Manually draw the ROI using the "polygon selection tool" located in the Toolbar.
Draw the ROI in the red channel in which the beta-cell nuclei are visible. Select the ROI such that it covers an area that is larger than a cell’s nucleus in order to include some of the cell’s cytoplasm. Ensure that the ROI position is consistent between frames and adjust the position if necessary.
Add the selected ROIs to the "ROI manager" by clicking on the "Add [t]" button. Select and add multiple ROIs to the ROI for obtaining data on multiple cells.
Next, from the "Analyze" menu, select "Set measurements". Select "Integrated Density" for specifying extraction of total fluorescence intensity within the area.
Shift to the green channel containing the GCaMP fluorescence and select "Multi Measure" in the "ROI Manager". NOTE: This will provide the intensity measurements for the cells throughout the time-series.
In case the ROI position needs to be adjusted due to movement of the islet, manually compile the intensity measurements in different frames. Copy and paste the values to a separate spreadsheet.
Obtain the timestamps of the image frames from "LSM Toolbox". Use "Apply stamps | Apply t stamps | File name | Dump to textfile" to obtain the timestamps. Save timestamps using the "Save as" option, or copy them into the spreadsheet.
Upon compiling the intensity values for all cells, perform the analysis one cell at a time or automatically (e.g., using Excel Formulas or R).
- Analyze individual cells in two steps.
- In the first step, calculate the fluorescence intensity above the baseline. To this end, calculate the baseline fluorescent intensity (F0) as the average of fluorescence intensities for the first 50 frames (5 mM glucose). Then subtract the baseline (F0) from the entire time-series (F – F0). NOTE: A few cells can show clear GCaMP oscillations under basal glucose, which typically continue following stimulation with higher concentrations. For such cells, it is only possible to estimate F0 by taking the mean intensity of the initial frames in which the cells showed a drop in fluorescence.
- In the second step of the analysis, obtain the final GCaMP fluorescence intensity by normalizing the fluorescence intensity. NOTE: This is performed to remove the differences between islets from different animals. Individual islets display varying levels of fluorescence emission upon glucose stimulation.
- Normalize the fluorescence intensity by dividing it with the highest intensity value. For this, calculate the peak intensity (Fmax – F0), and divide the baseline subtracted values by peak intensity to yield the final GCaMP fluorescence intensity (F – F0)/(Fmax – F0).
Discard the cells that do not exhibit a change in intensity following KCl stimulation, as they might be unhealthy or damaged.
For performing the analysis (steps 4.9–4.10) in R, use the R script (plotcelltrace.R) provided with this manuscript.
Representative Results
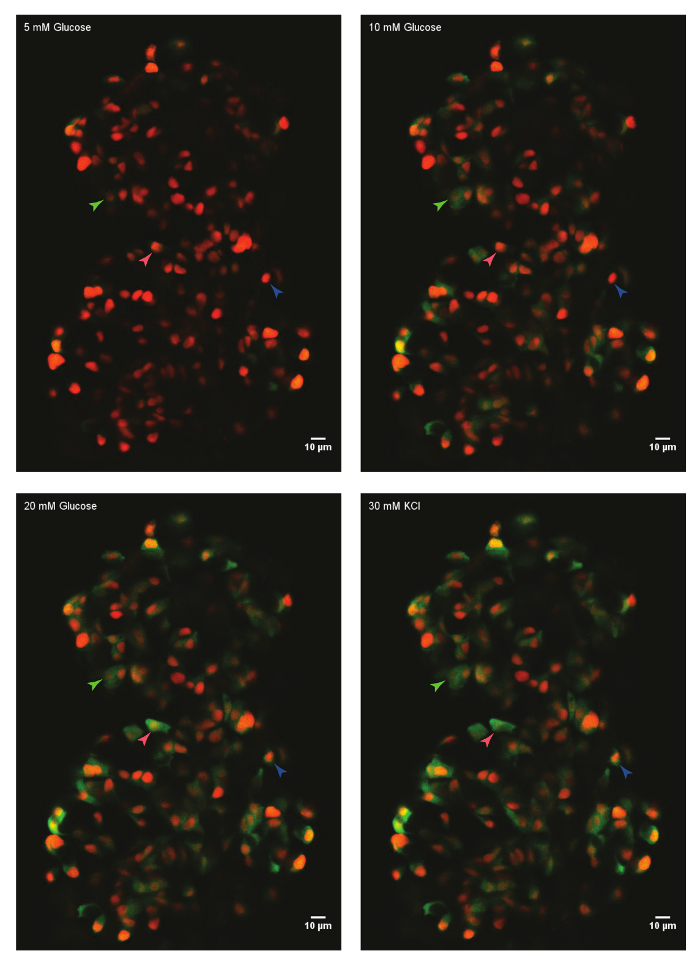
Using the protocol described above, the glucose responses of beta-cells in an islet from a 45 days post-fertilization (dpf) zebrafish were analyzed. For this, the primary islet was dissected from a euthanized animal and mounted in the fibrinogen-thrombin mold in a glass-bottom dish. The islet was immersed in HBSS containing 5 mM glucose. The glucose concentration was increased in a step-wise manner to 10 mM and 20 mM. The responses of beta-cells to the increasing glucose concentrations were recorded. Finally, beta-cells were depolarized using 30 mM KCl (Figure 1). The depolarization using KCl induces calcium entry in healthy beta-cells.
Using FIJI and a data analysis software, the GCaMP6s fluorescence intensity of individual beta-cells is extracted and normalized (Figure 2). As seen from the trace of fluorescence intensity, individual beta-cells display oscillations in GCaMP6s fluorescence upon glucose stimulation, which stops upon KCl stimulation. The technique provides a cellular resolution of the beta-cell's glucose-responsiveness and a window into their functionality.
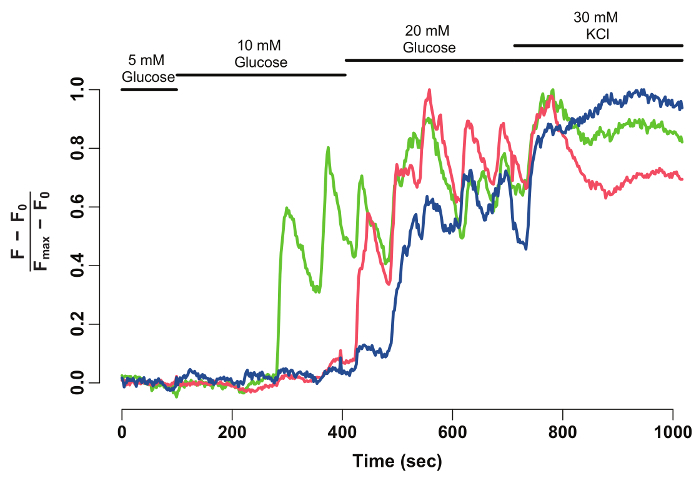
Figure 1: Ex vivo live-imaging of calcium influx using GCaMP6s in zebrafish beta-cells. A primary islet from Tg(ins:nls-Renilla-mKO2); Tg(ins:GCaMP6s) zebrafish (45 dpf) was mounted in fibrinogen-thrombin mold and incubated with 5 mM (basal) glucose. Beta-cells were labeled with a red nuclear marker, while the GCaMP6s fluorescence is present in the green channel. The islet was stimulated with a glucose-ramp consisting of sequential incubation with 10 mM and 20 mM D-glucose, and depolarized via the addition of 30 mM KCl. Arrowheads mark individual beta-cells whose activity was analyzed. Please click here to view a larger version of this figure.
Figure 2: Normalized GCaMP6s fluorescence intensity-trace for individual beta-cells. Normalized GCaMP6s fluorescence intensity-trace for the beta-cells marked with arrowheads in Figure 1. The X-axis denotes the time in seconds. At the top, bars depict the concentration of glucose and KCl in the HBSS medium. The Y-axis denotes the normalized fluorescence intensity during the time-series. For this, baseline intensity (F0) is calculated as the mean intensity during incubation in 5 mM glucose. This is subtracted from the entire time-series data (F - F0). The intensity-over baseline is normalized by the maximum intensity displayed by the cell (F - F0)/(Fmax- F0). The normalized trace shows an oscillatory response of beta-cells to glucose, which stabilizes when the cells are depolarized with 30 mM KCl. Please click here to view a larger version of this figure.
Discussion
Here we demonstrate a technique for the quantification of beta-cell glucose responsiveness at single-cell resolution. This is made possible by monitoring intracellular calcium concentration using a genetically-encoded calcium indicator, GCaMP6s. The beta-cell activity is captured ex vivo by mounting the islet in a fibrinogen-thrombin mold. A critical step of the protocol is the stability of the mold. Sufficient time needs to be given for the fibrinogen to dissolve in the HBSS solution. Without this, the mold does not polymerize sufficiently to provide stability during the imaging session. An islet mounted in fibrinogen-thrombin mold and immersed in cell culture media can remain viable for at least a week (data not shown). Alternatives to the fibrinogen-thrombin mold, such as low-melt agarose, can be utilized to mount the islet22. Another critical parameter is the dissection of the islet. During this step, the tissue surrounding the islet needs to be removed without injuring or poking the islet. A skillful dissection comes with practice.
A limitation of the imaging protocol is the restriction to one confocal plane of the islet. This is done to capture the dynamics of calcium influx within individual beta-cells. A Z-stack through the entire thickness of the islet leads to low imaging speeds and loss of oscillatory signal from individual cells. This limitation could be improved by using faster means of confocal-imaging such as spinning-disk microscopy, to enable capturing the calcium dynamics in 3-dimensions. Another frontier would be in vivo calcium imaging12. The transparent nature of zebrafish embryo or the use of pigment-less strains of zebrafish adults23 could open the possibility for in vivo imaging in the future.
The imaging of beta-cell activity at high spatial and temporal resolution enables to investigate the functional heterogeneity among individual beta-cells. This approach can help shed light on the existence of beta-cell sub-populations. Recently, multiple studies have shown the existence of sub-populations in the nominally homogenous beta-cells24,25,26. Ex vivo imaging can be combined with genetic reporters to characterize of the sub-population's response to glucose. Additionally, combining the imaging setup with pharmacological stimulation can allow for screening of compounds that could improve beta-cell functionality.
In summary, the technique presented here allows quantification and comparison of the glucose responsiveness for individual beta-cells. It provides a direct window into beta-cell functionality, an important parameter in development of diabetes.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
We thank members of the Ninov lab for comments on the manuscript, members of Center for Regenerative Therapies Dresden (CRTD) fish and microscopy facility for technical assistance. N.N. is supported by funding from the DFG-CRTD, Cluster of Excellence at TU-Dresden, German Research Foundation (DFG) and the German Center for Diabetes Research (DZD).
References
- Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- Ogurtsova K, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Pipeleers DG. Heterogeneity in pancreatic beta-cell population. Diabetes. 1992;41(7):777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B, et al. Glucose induces oscillatory Ca2+ signalling and insulin release in human pancreatic beta cells. Diabetologia. 1994;37(Suppl 2):S11–S20. doi: 10.1007/BF00400821. [DOI] [PubMed] [Google Scholar]
- Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem. 1994;269(12):8749–8753. [PubMed] [Google Scholar]
- Wollheim CB, Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984;259(4):2262–2267. [PubMed] [Google Scholar]
- Pace CS, Price S. Electrical responses of pancreatic islet cells to secretory stimuli. Biochem Biophys Res Commun. 1972;46(4):1557–1563. doi: 10.1016/0006-291x(72)90785-1. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Ishida H, Nagamatsu S. Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy: distinct behavior of granule motion in biphasic insulin release. J Biol Chem. 2002;277(6):3805–3808. doi: 10.1074/jbc.C100712200. [DOI] [PubMed] [Google Scholar]
- Soria B, Martin F. Cytosolic calcium oscillations and insulin release in pancreatic islets of Langerhans. Diabetes Metab. 1998;24(1):37–40. [PubMed] [Google Scholar]
- Kenty JHR, Melton DA. Testing pancreatic islet function at the single cell level by calcium influx with associated marker expression. PLoS One. 2015;10(4):e0122044. doi: 10.1371/journal.pone.0122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Rietdorf K, Collins T, Walker S, Sanderson M. Ca2+-sensitive fluorescent dyes and intracellular Ca2+ imaging. Cold Spring Harb Protoc. 2013;2013(2):83–99. doi: 10.1101/pdb.top066050. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson D, Anderson RM, Beinat M, Stainier DYR. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc Natl Acad Sci U S A. 2009;106(35):14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124(3):218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S, Stainier DYR, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3(6):948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, et al. Different developmental histories of beta-cells generate functional and proliferative heterogeneity during islet growth. Nat Commun. 2017;8(1):664. doi: 10.1038/s41467-017-00461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2016. Available from: https://www.r-project.org/
- Friedman RS, et al. An evolving autoimmune microenvironment regulates the quality of effector T cell restimulation and function. Proc Natl Acad Sci. 2014;111(25):9223–9228. doi: 10.1073/pnas.1322193111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader E, et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature. 2016;535(7612):430–434. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- Dorrell C, et al. Human islets contain four distinct subtypes of β cells. Nat Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NR, et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016;24(3):389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


