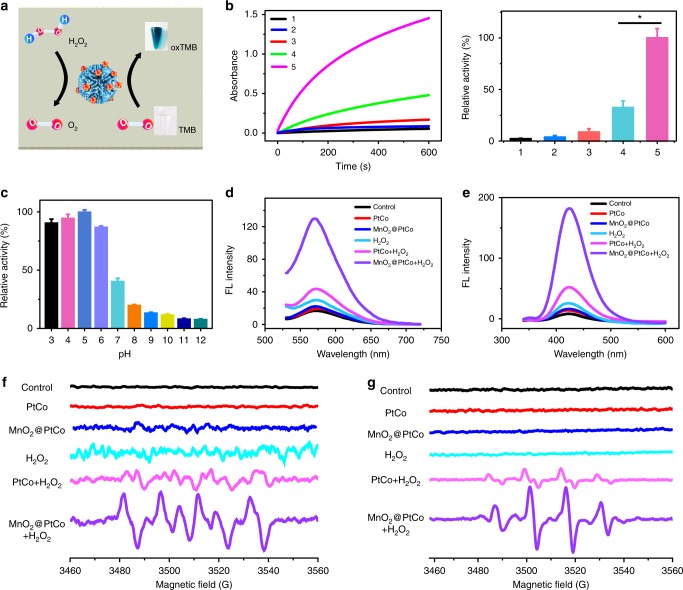
Fig. 3.
Multi-enzyme activity and catalytic mechanism of MnO2@PtCo nanoflowers in hypoxia. a Schematic illustration of catalytic reactions of MnO2@PtCo nanoflowers in the hypoxic H2O2 condition. b The oxidase-like activity of various nanomaterials by monitoring the absorption of TMB at 652 nm and the qualitative analysis of their catalytic activity in the hypoxic H2O2 (100 μM) environment. 1: H2O2 alone; 2: PtCo; 3: MnO2@PtCo; 4: PtCo + H2O2; 5: MnO2@PtCo + H2O2. Asterisks indicate significantly differences (*P < 0.01, **P < 0.005, ***P < 0.001), analyzed by unpaired Student’s two-sided t test. c The influence of pH on the catalytic ability of MnO2@PtCo nanoflowers. d Fluorescence spectra of hydroethidine incubated with MnO2@PtCo nanoflowers in the hypoxic H2O2 (100 μM) condition to demonstrate the presence of O2•−. e Fluorescence spectra of TA incubated with MnO2@PtCo nanoflowers in the hypoxic H2O2 (100 μM) condition to demonstrate the generation of •OH. f ESR spectra of BMPO/•OOH adducts from different groups in the hypoxic H2O2 (100 μM) condition upon addition of DMSO. g ESR spectra of BMPO/•OH adducts were collected from different samples in the hypoxic H2O2 (100 μM) condition upon addition of SOD. Data were presented as mean ± s.d. (n = 5)