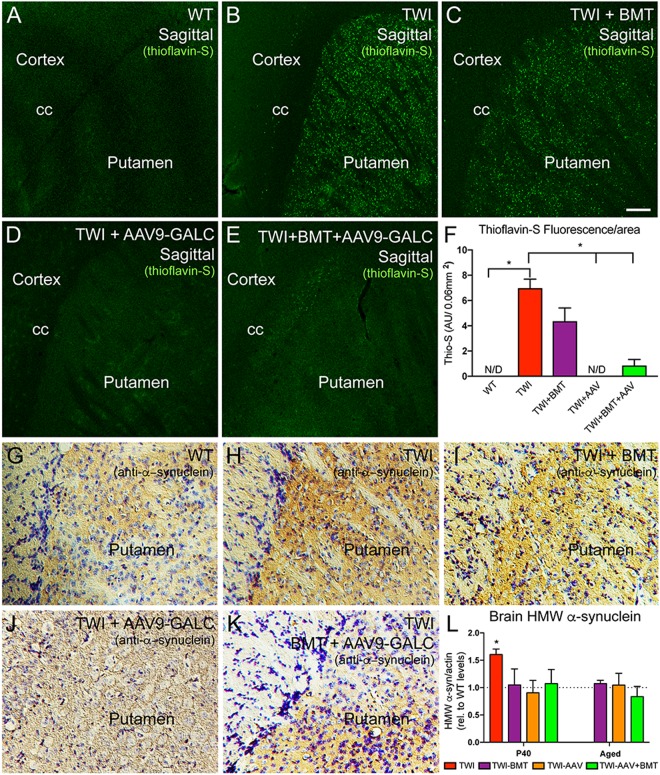
Figure 2.
Neuronal thioflavin-S aggregates and high-molecular-weight α-synuclein aggregates are greatly reduced upon AAV9-GALC gene therapy. Thio-S+ protein aggregates in TWI (B), previously shown to be intra-neuronal and partly comprised of α-synuclein1, were eliminated at P40 after neonatal gene therapy using AAV9-GALC vectors alone31 (D) or in combination with bone marrow transplantation (BMT + AAV9-GALC) (E), but not by BMT alone (C). WT comparison in (A). Images show anatomical regions encompassing deep cerebral cortex and the putamen. cc, corpus callosum. Thio-S+ aggregates (fluorescent units per area) remained largely eliminated in AAV9-GALC and BMT + AAV9-GALC brains (F). *p < 0.05, compared to WT, ANOVA; n = 3–4 mice per group). (G,K) Gene therapy with AAV9-GALC greatly normalized α-synuclein levels in the cortex/putamen region (compare J and K with H). Sections were stained with diamino-bencidine for α-synuclein and counterstained with toluidine blue. (L) Total brain lysates from young (P40) and long-surviving (>P200) TWI, AAV9-GALC treated TWI and control mice31 were immunoblotted for α-synuclein, and high-molecular-weight bands (~30 to ~100 kDa) were quantified by optical densitometry. Results are expressed as fold levels over basal levels in WT brains. *p < 0.05, ANOVA; n = 3–4 mice per group. Scale bar: 200 μm.