Figure 1:
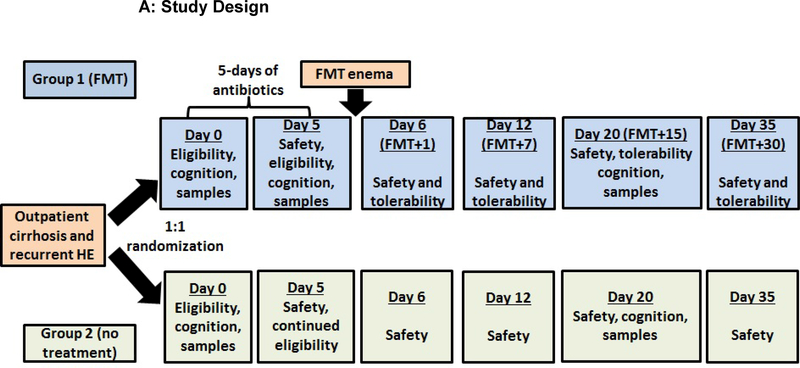
Patient flow and study design;
Figure 1A: Flow chart of study design; patients were followed for 150 days after enrollment. At day 5 after randomization (post-antibiotics in the FMT arm), blood, urine and stool was collected from all participants and adverse events (AE), new medications and diagnoses as well as adherence to the original diet was documented. All participants were seen at day 6 after randomization (day 1 post-FMT in the FMT arm) where a solicited/unsolicited AE assessment, safety laboratories (MELD score, liver function tests, CBC), urine and stool was collected. Similarly, at days 12 and 20 post-randomization, we performed safety laboratories, collected blood, urine and stool, and evaluated AEs. At day 20, both cognitive tests were re-administered. At day 35, we collected safety laboratories and evaluated AEs. At day 150, SAE assessment was conducted.
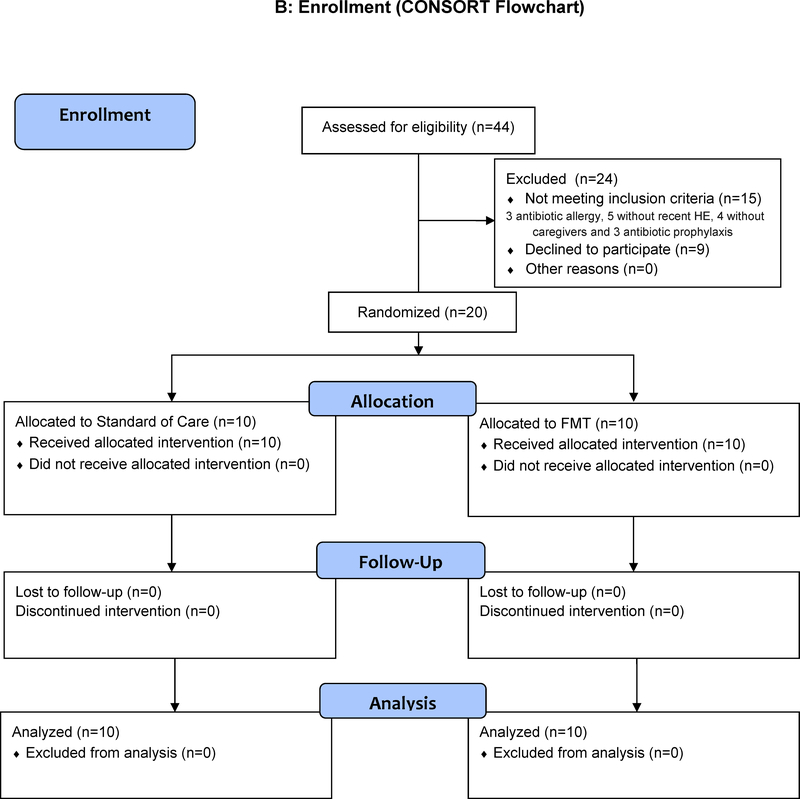
Figure 1B: CONSORT Flow chart showing that there were no dropouts and all subjects were followed throughout the study