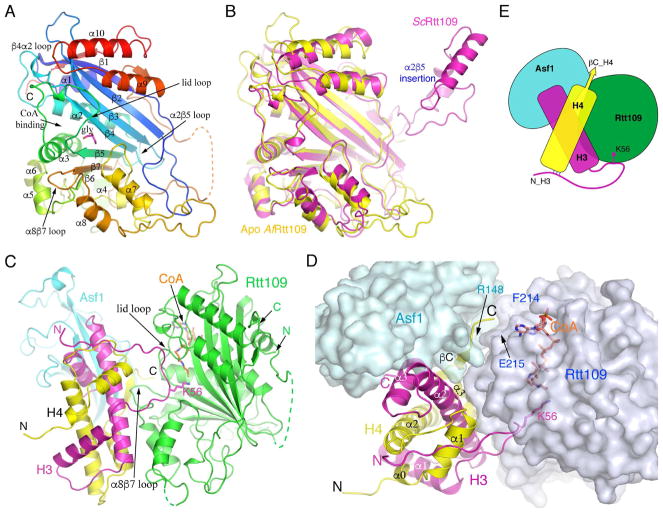
Figure 2. Structures of AfRtt109 and its complex with AfAsf1-H3-H4.
(A) Apo structure of AfRtt109 colored in rainbow spectrum. A glycerol molecule (magenta) sits near the active site and is shown in a stick model. (B) Comparison of the apo AfRtt109 structure (yellow) with the structure of ScRtt109 (magenta) from its complex with Vps75 (PDB id: 3Q66). The nonconserved α2β5 insertion in ScRtt109 (see Figure 1A) important for Vps75 binding is indicated. (C) Front view of the structure of AfRtt109 (green) in complex with AfAsf1 (cyan), histones H3 (magenta), H4 (yellow) and CoA. (D) A top view of the structure with AfRtt109 and AfAsf1 shown in a semi-transparent surface representation colored in pale blue and light green, respectively. The sidechains of H3K56, Glu215 of AfRtt109 and Arg148 of AfAsf1 are superimposed and labeled. (E) A cartoon drawing schematizing protein-protein interactions of the AfRtt109 complex. See also Figures S1–S3; Table S1.