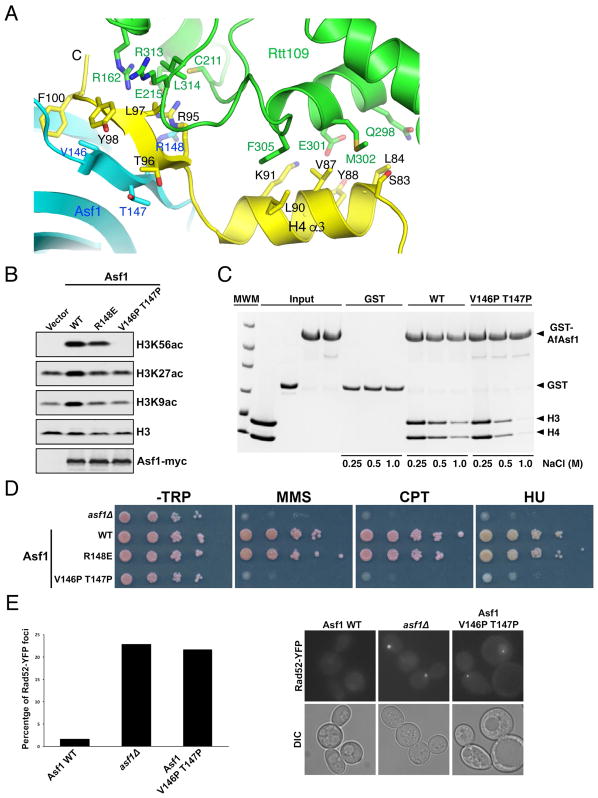
Figure 4. Asf1 stimulates H3K56 acetylation through stabilization of the C-terminal end of H4.
(A) The interface between AfAsf1 (cyan), H4 (yellow) and AfRtt109 (green). Involved residues are labeled. (B) Western blot detection of H3K56, H3K27 and H3K9 acetylation in budding yeast cells expressing indicated Asf1 mutants. (C) GST-pulldown of WT and the V146P T147P mutant of AfAsf1 in the presence of H3-H4 with at the indicated salt concentrations detected by coomassie blue staining. (D) Cell growth analysis with ten-fold serial dilutions of budding yeast cells expressing the indicated Asf1 proteins in the absence and presence of indicated DNA-damaging agents. (E) Live cell fluorescence images showing that the Asf1 V146P T147P mutant budding yeast cells exhibit a higher percentage (left panel, quantification from two independent experiments) of cells containing spontaneous Rad52-YFP foci (right panel, representative images) than WT cells. See also Figures S4, S5; Tables S2, S3.