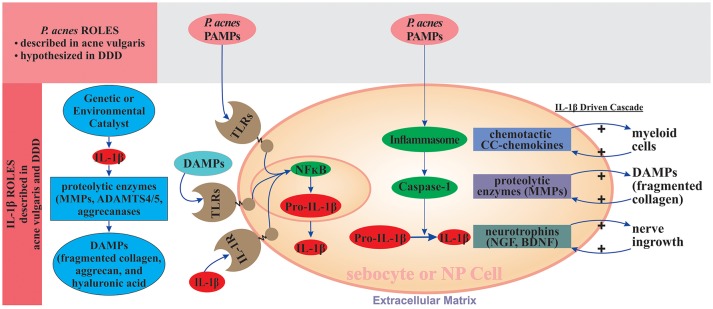
Figure 3.
IL-1β driven degenerative state in acne vulgaris and degenerative disc disease. Following the initiating event, sebocytes and NP and AF cells promote production of IL-1β. IL-1β signaling facilitates extracellular matrix degradation through induction of proteolytic enzymes, including MMPs and ADAMTS4/5. P. acnes PAMPs and extracellular matrix degradation products DAMPs (e.g., fragmented collagen, aggrecan, and hyaluronic acid) bind to TLRs, which activate NF-κB, leading to the synthesis of pro-IL-1β; P. acnes PAMPs also activate inflammasomes (NLRP3) and caspase-1, which convert pro-IL-1β to IL-1β. IL-1β stimulates production of CC-chemokines that recruit myeloid cells and proteolytic matrix metalloproteases (MMPs) that degrade the ECM. Positive feedback loops arise when IL-1β binds to IL-1R and when extracellular matrix degradation products (DAMPs, e.g., fragmented collagen) activate TLRs. Both binding events stimulate IL-1β production. Inflammatory signaling is amplified since IL-1β is not only NF-κB target gene, but also its activator through binding to IL-R1. The nerve ingrowth into the degenerated disc, usually accompanied by presence of the annular fissures eventually herniation, is considered a main source of nociception and thus low back and/or radicular pain. IL-1β induces the expression of neurotrophins like NGF and BDNF supporting the nerve. ADAMTS-4/5, a disintegrin and metalloproteinase with thrombospondin motifs 4/5; AF, annulus fibrosus; BDNF, brain-derived neurotrophic factor; DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns; DDD, degenerative disc disease; ECM, extracellular matrix; IL-1R, interleukin-1 receptor; MMPs, matrix metalloproteinases; NF-κB, nuclear factor κB; NGF, nerve growth factor; NP, nucleus pulposus; TLRs, Toll-like receptors.