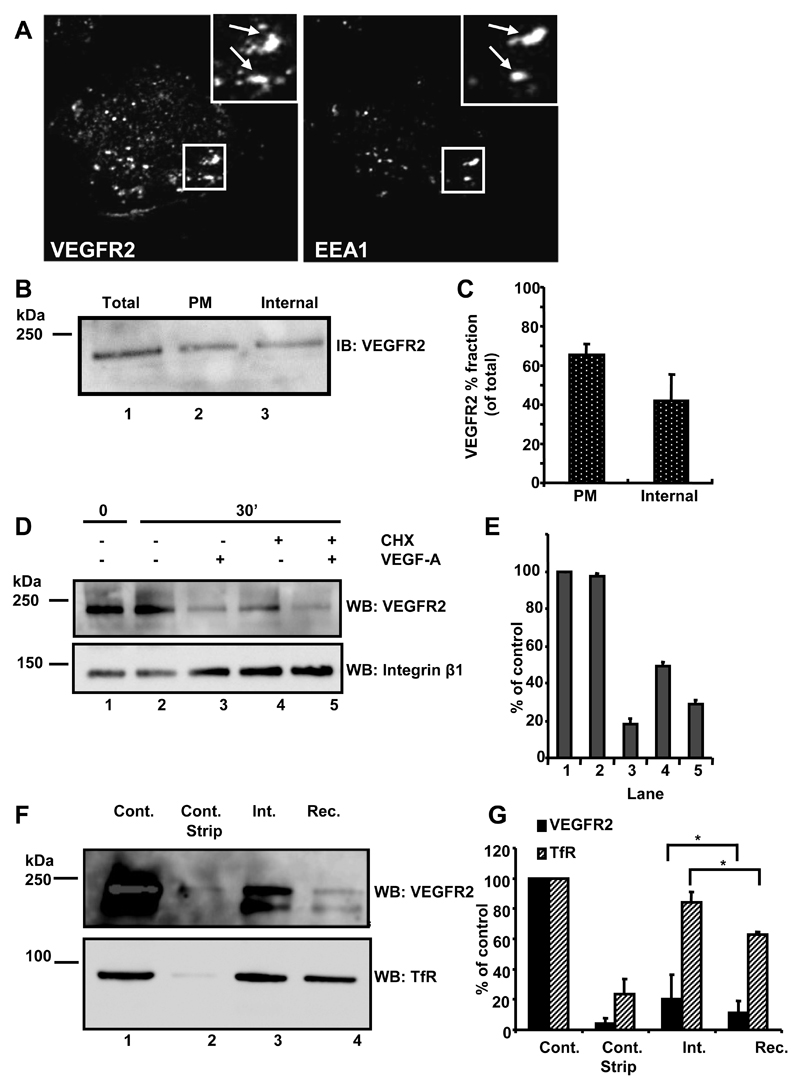
Fig. 1.
VEGFR2 undergoes endocytosis and recycling. (A) Quiescent HUVECs were fixed, permeabilized and labeled with goat anti-extracellular domain VEGFR2 and rabbit anti-EEA1. Bound primary antibodies were visualized using species-specific AlexaFluor-conjugated secondary antibodies. Insets show 2-fold magnification of indicated areas and arrows indicate co-localization of both proteins. Data shown are representative of three independent experiments. (B) Biotinylated and non-biotinylated proteins from quiescent HUVECs were probed by immunoblotting (IB) for relative total VEGFR2 (total, lane 1) with parallel analysis of the biotinylated plasma membrane pool (PM, lane 2). The remaining supernatant represented intracellular endosomal VEGFR2 (internal, lane 3). (C) Quantification of cell surface biotinylation and immunoblotting (error bars denote ±SEM; n=3). (D) Starved endothelial cells were treated with cycloheximide (CHX; 2 hr) to block new protein synthesis where indicated. VEGF-A stimulation for 0 or 30 min was followed by cell surface biotinylation, lysis and immunoblotting using goat anti-VEGFR2 or mouse anti-β1 integrin. (E) Quantification of representative datasets shown in panel (D) (error bars denote ±SEM; n=3). (F) Starved quiescent endothelial cells were biotinylated at 4°C and either lysed directly (control) or subjected to reducing cleavage to strip the biotin label (control strip). Other samples were biotinylated at 4°C, and then incubated at 37°C for 10 min to allow internalisation prior to reduction and lysis (int.). For recycling analysis (rec.), samples were incubated for a further 20 min at 37°C before reduction, lysis, isolation of biotinylated proteins and immunoblotting sequentially with goat anti-VEGFR2 followed by mouse anti-TfR. (G) Quantification of representative datasets shown in panel (F) (error bars denote ±SEM; n=3).