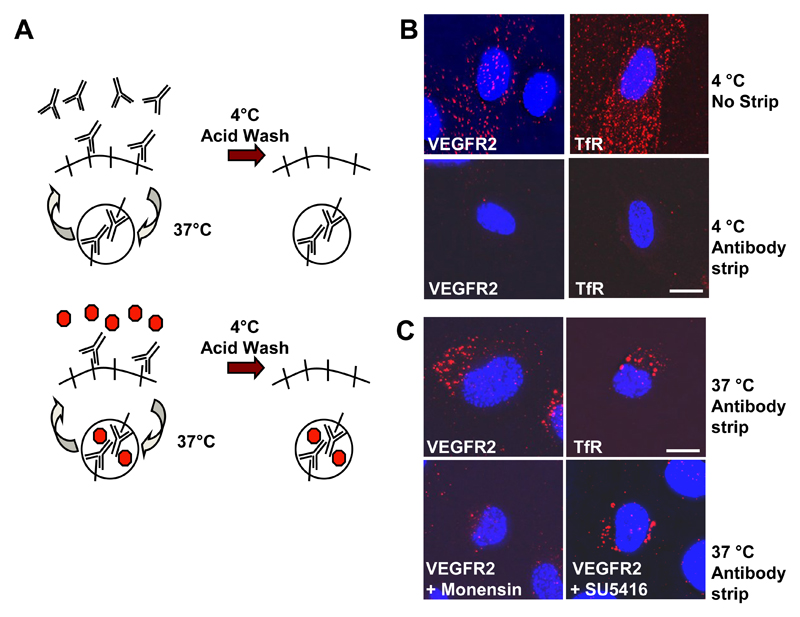
Fig. 2.
VEGFR2 recycling is independent of tyrosine kinase activity. (A) Schematic outline of an antibody-based assay to monitor receptor recycling between the plasma membrane and internal compartment(s). (B) Endothelial cells were labeled with goat anti-VEGFR2 or mouse anti-transferrin receptor (TfR) for 1 h at 4°C. Bound cell surface antibodies were either stripped by acid wash (antibody strip) or left bound to the protein (no strip). Cells were subsequently labeled for a further 1 h at 4°C with AlexaFluor-594-conjugated anti-sheep or Texas Red-conjugated anti-mouse IgG. Accessible cell surface antibody complexes were again removed from stripped samples by acid wash and samples were fixed and labeled with a DNA stain (DAPI; blue) before microscopy. Bar, 10 μm. (C) Direct recycling assay for cells treated with anti-VEGFR2 or anti-TfR antibodies at 37°C for 30 min. Cells were also pre-treated with either 20 μM monensin (endosome-to-plasma membrane recycling inhibitor) or 5 μM SU5416 (VEGFR tyrosine kinase inhibitor)and labeled with anti-VEGFR2. Results are representative of three independent experiments. Bar, 10 μm.