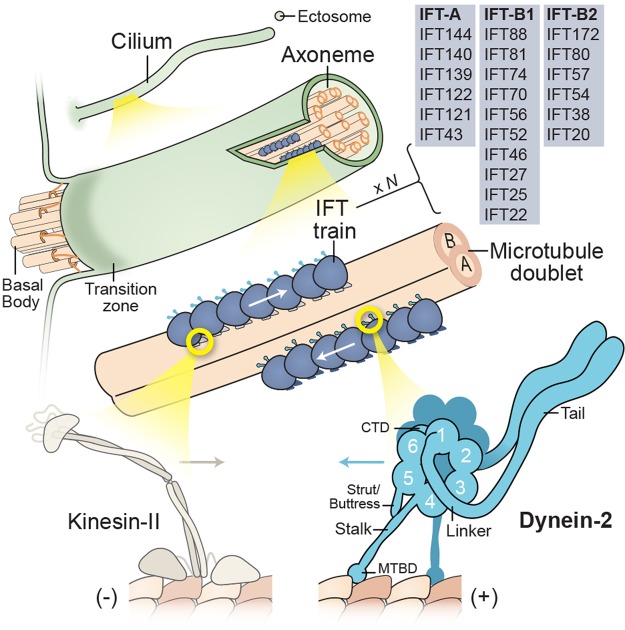
Box 1. Cilia and intraflagellar transport.
Cilia fall into two broad classes: motile and non-motile. Motile cilia beat with a wave-like motion to either propel cells, such as sperm and protozoa, or generate flow over the cell surface. Conversely, a non-motile primary cilium is present on almost every cell type in the human body. A widespread view was that primary cilia represented nonfunctional vestigial structures. However, landmark discoveries recast primary cilia as the ‘signaling antenna’ of the cell [149–151]. For example, mutations causing polycystic kidney disease were linked to an IFT subunit (IFT88) and a shortened cilia phenotype, supporting a sensory role for kidney primary cilia [152]. Hedgehog signaling, an important pathway for embryonic patterning, was found to depend on IFT proteins in mice [153] and involve dynamic localization of the receptor Smoothened to and from the ciliary membrane [154]. Other signaling components, involved in processes as diverse as sight, smell, taste, and appetite control, localize within cilia [150]. Moreover, receptor-containing ectosomes have recently been found to be secreted from the tip of the cilium to modulate signaling [155–158]. The core of all cilia is the axoneme, a cylindrical array of typically nine microtubule doublets that extends from the basal body. Motile axonemes normally also have a central pair of microtubules, as well as periodic arrays of axonemal dyneins and regulatory complexes that co-ordinate ciliary beating [159]. The axoneme is covered by a ciliary membrane that is continuous with the plasma membrane but distinct in protein and lipid content. During ciliary growth and maintenance, new subunits are incorporated at the ciliary tip, where the microtubule plus ends (+) reside. A diffusion barrier separates the ciliary volume and the cytoplasm [160,161]. This selective barrier involves the ‘transition zone’, a region immediately distal to the basal body characterized by Y-shaped links between the doublets and the ciliary membrane. Anterograde IFT, powered by kinesin-II motors, moves cargoes synthesized in the cytoplasm through the transition zone and toward the tip of the cilium. Conversely, dynein-2 returns cargoes to the cell body. Both motors associate with IFT trains, polymeric arrays involving two sub-complexes, IFT-A and IFT-B (consisting of IFT-B1 and IFT-B2) [162,163]. Genetically, IFT-B proteins tend to be critical for anterograde IFT and ciliogenesis, while IFT-A proteins are typically linked with retrograde IFT. However, the functions of IFT-A and -B are not so simply separated, as IFT-B proteins can be involved in cargo export, while IFT-A proteins are required for ciliary entry of a subset of membrane proteins via the adaptor protein TULP3 [164]. Structurally, dynein-2 comprises a tail domain and a motor domain containing the linker, a ring of six AAA+ modules (1–6), a coiled-coil stalk with the MTBD at its tip, a shorter coiled-coil strut/buttress, and a C-terminal domain (CTD).