Figure 3. Mucin-type O-GalNAc glycosylation changes seen in breast cancer lead to increase in tumour growth and progression through a number of mechanisms.
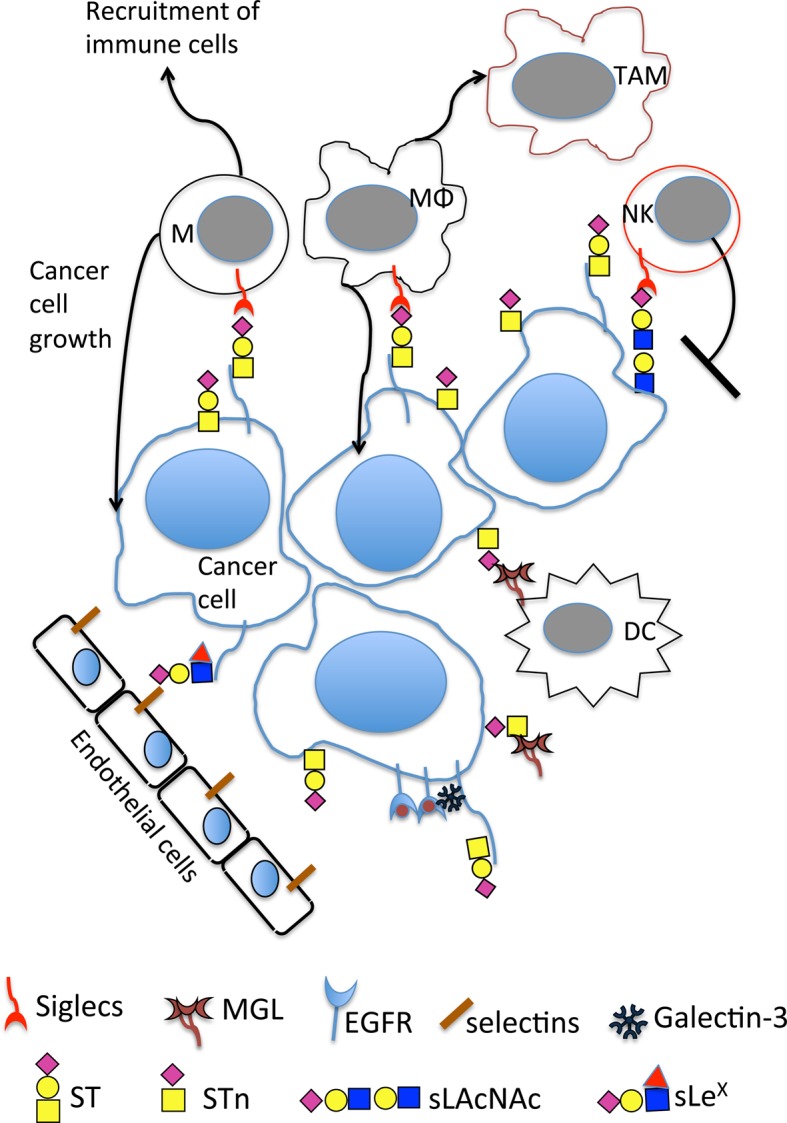
O-GalNAc changes lead to novel interactions with lectins on immune cells including the binding of sialylated glycans to siglecs on monocytes, macrophages and NK cells. This includes the binding of MUC1-ST to siglec-9 on monocytes and macrophages and the binding of sialylated LacNAc (which can be carried on core 1 and core 2 branches) to siglec-7 on NK. Binding of Tn and STn to MGL on dendritic cells and macrophages is also observed. Expression of sLeX can lead to binding to selectins on endothelial cells, and different core glycans can dictate how cancer cells respond to EGF binding. M, monocytes; MΦ, macrophages; TAM, tumour-associated macrophages; NK, natural killer cells; DC, dendritic cells; MGL, macrophage galactose-specific lectin; EGFR, epidermal growth factor receptor. Symbols for the glycans are as in Figure 1 with the addition of the red triangle for fucose.
