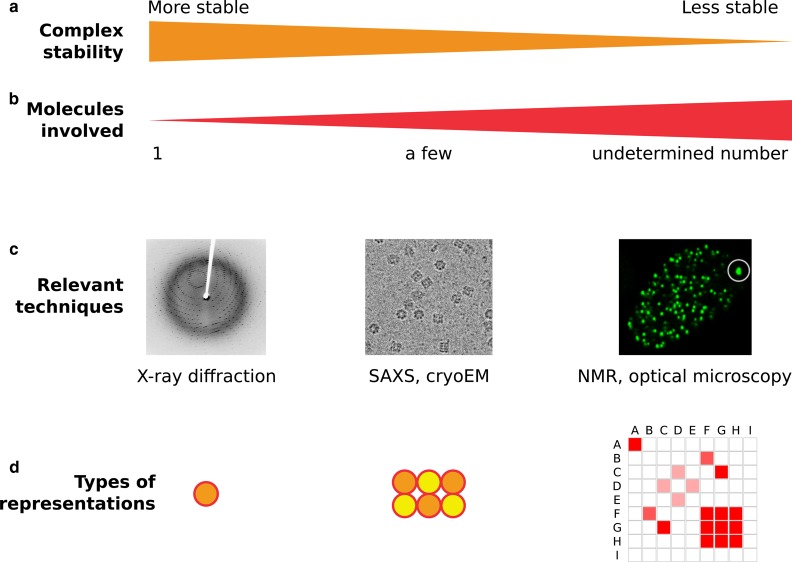
Figure 1. Diversity of weak interactions and example techniques to study them.
Biological interactions cover a wide spectrum in terms of complex stability (a) and number of molecules involved (b). This spectrum spans from stable protein complexes that can be purified and further imaged by techniques such as X-ray diffraction (left) to very labile, transient interactions that can involve thousands of proteins in vivo, whereas none of the interactions can be captured by traditional biochemistry (right). As one goes from one end of the spectrum to the other end, distinct sets of techniques (c) and types of representations (d) are needed. For instance, as the valency of interactions increases from a few strongly interacting partners to many weakly interacting partners, new graphical representations are needed, since traditional schematics representing macromolecular complexes whose stoichiometry is known as the juxtaposition of monomers (center) become difficult to read when depicting one protein weakly interacting with dozen of partners (right). In that case, matrices of pairwise interactions between proteins A-I might be more relevant. SAXS: Small-Angle X-Rays Scattering, NMR: Nuclear Magnetic Resonance.