Figure 2.
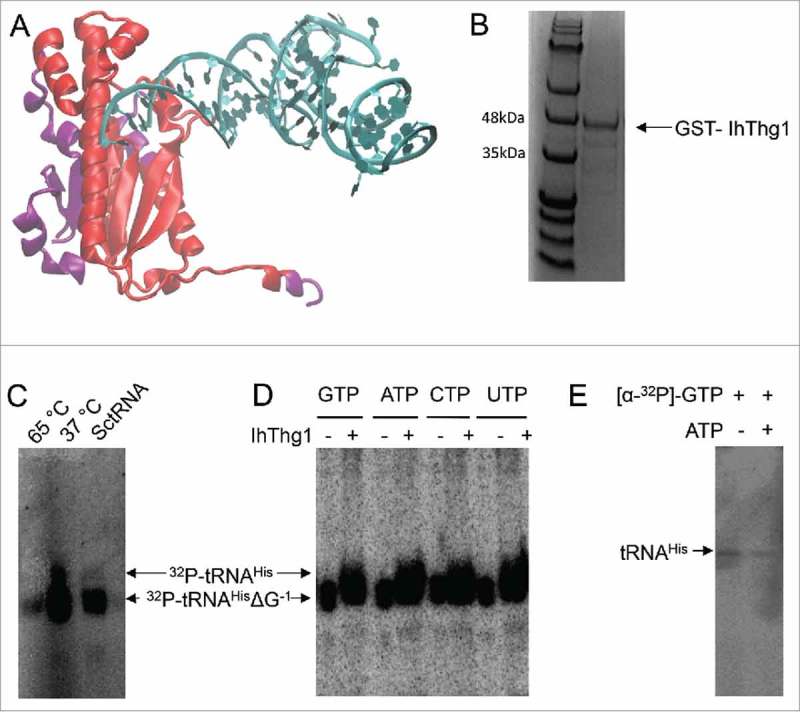
The naturally shortened I. hospitalis enzyme is enzymatically active. (A) Projection of the IhThg1 domains onto CaThg1 (pdb 3wc1). Sections missing in IhThg1 are depicted in purple. (B) IhThg1 was purified as a GST-fusion protein to apparent homogeneity. (C, D, and E) Autoradiography of IhThg1 reaction products separated on a 12% denaturing polyacrylamide gel with 8M Urea. For enzyme activity assays, proteins were incubated with 3’-monophosphorylated tRNAHisΔG−1 transcripts and the indicated nucleotides. Successful nucleotide addition results in a shift of the radiolabelled transcript or the appearance of a radiolabelled transcript. (C) IhThg1 incubated with GTP, ATP, and labelled 3’-32P -tRNAHisΔG−1 displays enzyme activity at 37 °C, but not at 65 °C, and exhibited reduced activity with S. cerevisiae tRNAHisΔG−1. (D) IhThg1 was incubated with labelled E. coli 3’-32P-tRNAHisΔG−1 and individual nucleotides GTP, ATP, CTP, and UTP and displayed nucleotidyltransferase activity with all four nucleotides. (E) IhThg1 was incubated with tRNAHisΔG−1 and [α-32P]-GTP in the absence and presence of ATP. IhThg1 enzyme activity is independent of ATP.
