Figure 6.
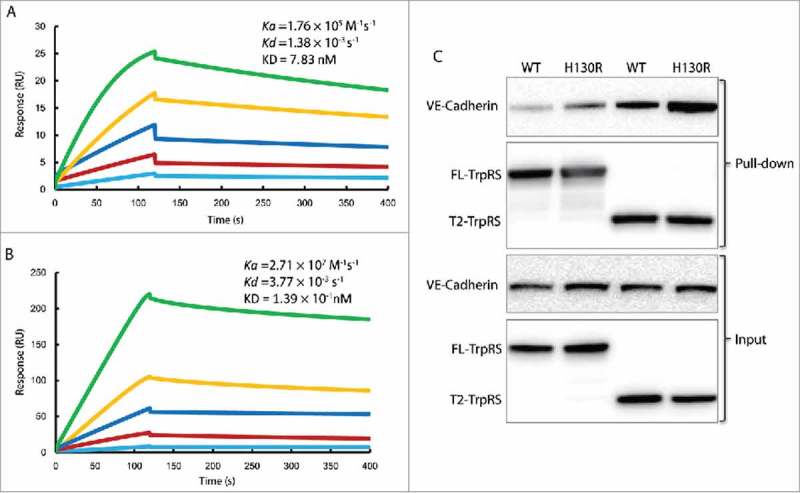
H130R substitution enhances the interaction between T2-TrpRS and VE-cadherin. (A) The fitted sensorgram of the binding between wild-type (WT) T2-TrpRS and EC1-2 domain of VE-cadherin by surface plasmon resonance. The response unit is plotted over 400 s that illustrates the association and dissociation of the complex. The concentration of WT T2-TrpRS is 2-fold increased from 12.5 nM to 200 nM. (B) The fitted sensorgram of the binding between H130R T2-TrpRS and EC1-2 domain of VE-cadherin. The response unit of H130R T2-TrpRS at concentration from 0.78 nM to 25 nM is plotted over 400 s. (C) In vitro pull down assay to test the interaction between TrpRS and endogenous VE-cadherin. The melanoma C8161 cells expressed VE-cadherin was pulled down by 6xHis tagged FL- and T2-TrpRS (WT and H130R), and immune-blotted by rabbit anti VE-cadherin. The TrpRS variants are blotted with HRP-conjugated 6xHis antibodies.
