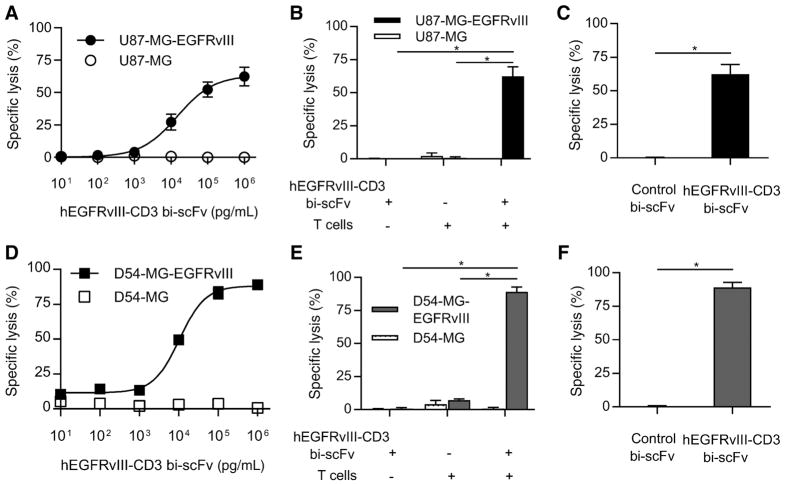
Figure 4.
hEGFRvIII-CD3 bi-scFv redirects T cells to specifically lyse EGFRvIII-positive glioma. A, Tumor cell lysis was assessed against two different glioma cell lines (EGFRvIII-positive and -negative for both). A dose-response–based increase in tumor cell lysis was observed against U87-MG-EGFRvIII glioma cells while no increase in lysis of U87-MG was observed with increasing bispecific antibody concentrations. B, The ED50 for the U87-MG-EGFRvIII cell line was 15,881 pg/mL. Both T cells and drug were necessary for significant specific lysis of U87-MG-EGFRvIII cells (P = 0.0033 and P = 0.0032, respectively) while the combination failed to induce any significant increase in lysis of target-negative cells compared to groups without drug or target antigen (P = 0.391 and 0.195, respectively). C, Significant drug-induced specific lysis of EGFRvIII-positive glioma was also observed when compared with that induced by control bi-scFv that binds to EGFRvIII but not CD3 (P = 0.0033). D–F, The same trends were observed against a second malignant glioma cell line both with and without EGFRvIII (D54-MG). The estimated ED50 for the D54-MG-EGFRvIII cell line was 10,595 pg/mL. Likewise, both T cells and drug were necessary for significant specific lysis of D54-MG-EGFRvIII cells (P < 0.0001 in both cases) while the combination failed to induce any significant increase in lysis of target-negative cells compared with groups without drug or target antigen (P = 0.1011 and 0.4659, respectively). Significant drug-induced specific lysis was observed against the D54-MG-EGFRvIII cell line when compared with that induced by control bi-scFv (P = 0.0001). Among each of the EGFRvIII-positive cell lines tested, the number of EGFRvIII receptors per cell was either similar to or an order of magnitude less than the number of EGFRvIII receptors per cell found among the patient-derived cells used in this study that endogenously express the target antigen (see Supplementary Table S2).