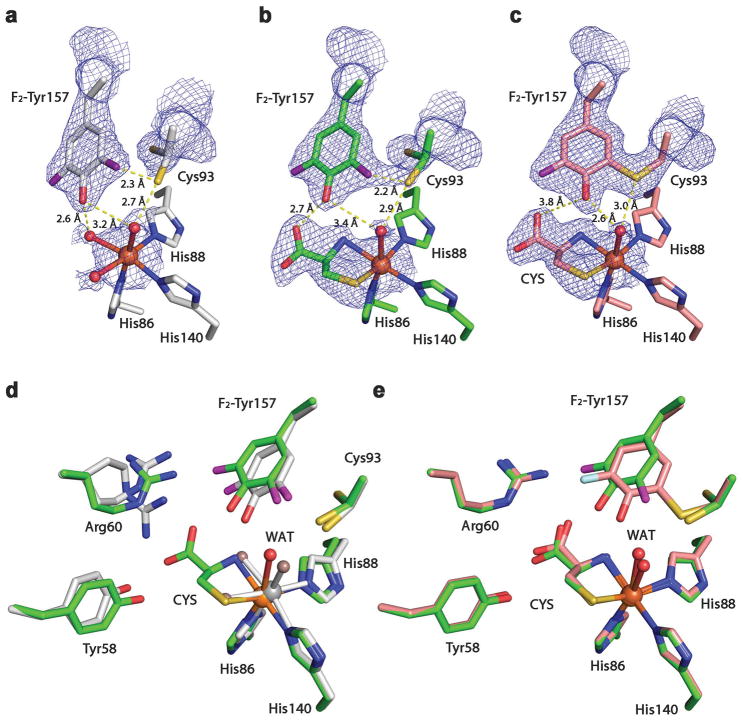
Figure 3. Crystal structures of F2-Tyr157 CDO.
2Fo – Fc electron density was contoured at 1.2 σrms, with potential H-bond interactions (broken lines) shown. a, The active site of the 100% uncrosslinked F2-Tyr157 CDO structure in the substrate-free form. b, The active site of the 100% uncrosslinked F2-Tyr157 CDO structure in the substrate-bound form. c, The active site of the mature F2-Tyr157 CDO structure in the substrate-bound form. d, Overlaid active site of the 100% uncrosslinked F2-Tyr157 CDO structure in the substrate-free (2.40 Å resolution, grey) and substrate-bound (2.10 Å resolution, green) forms. e, Superimposition of the substrate-bound mature F2-Tyr CDO (1.95 Å resolution, pink) and 100% uncrosslinked structures (green). The L-cysteine substrate is labeled as CYS. The omit Fo-Fc electron densities for the water ligands (WAT) and the L-cysteine are shown in Supplementary Figure 2.