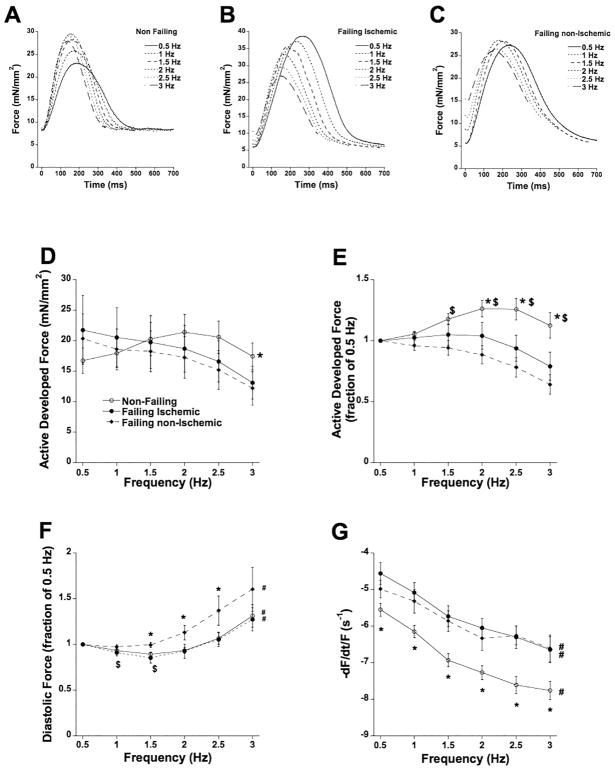
Figure 5.
A: Twitch contractions of a trabecula from a non-failing heart (#118258) at 6 frequencies, spanning the in vivo range showing a positive force-frequency relationship. B: Twitch contractions of a trabecula from a failing ischemic heart (#100714) at 6 frequencies showing a blunted/negative force-frequency relationship. C: Twitch contractions of a trabecula from a failing non-ischemic heart (#437918) at 6 frequencies showing a blunted/negative force-frequency relationship. D: Frequency-dependent regulation of force was different between failing and non-failing myocardium. The interaction of force and frequency was significantly (P<0.01) more positive in non-failing myocardium (n=33). E: Same data as panel A, plotted to each muscle’s force at 0.5 Hz to allow equal weight of each muscle in the statistical analysis. At all frequencies, force in non-failing trabeculae exceeded the force at the 0.5 Hz baseline. $ P<0.05 vs. non-ischemic failing trabeculae (n=8); * P<0.05 vs. ischemic failing trabecular (n=13). F: Relative to diastolic force at 0.5 Hz, diastolic force decreases ($, P<0.05) in the non-failing and failing ischemic group. The relative increase in diastolic force was significantly higher (*, P<0.05) in the failing non-ischemic group. In all groups, at 3 Hz the diastolic force was significantly higher than the 0.5 Hz baseline (#, P<0.05). G: The maximal kinetic rate of relaxation was significantly impacted by frequency in all groups (#, P<0.0001) and in addition was significantly faster (*, P<0.05) in non-failing trabeculae versus both failing groups.