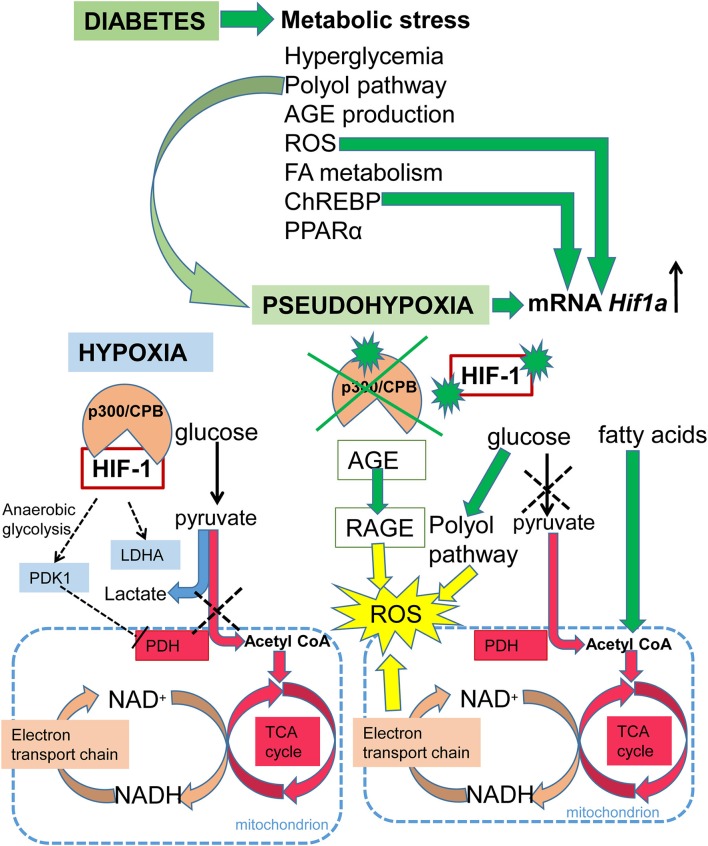
Figure 3.
Schematic summary of HIF-1 signaling in hypoxia and diabetes. Under normoxia, glucose is metabolized to pyruvate and then the pyruvate dehydrogenase complex converts pyruvate to acetyl-CoA, which enters the TCA cycle, and NADH and FADH2 are generated. Electrons derived from NADH and FADH2 are utilized by the electron transport chain to generate ATP. Under hypoxia, HIF-1 activates glycolytic genes as a critical step of metabolic adaptation to hypoxia. HIF-1 induces anaerobic glycolysis by mediating the expression of pyruvate dehydrogenase kinase 1 (PDK1), which inhibits pyruvate dehydrogenase (PDH) and induces conversion of pyruvate to lactate. Under diabetes, metabolic stress is induced by: an increased ratio of NADH/NAD+ due to an increased flux of glucose through the polyol pathway resulting in pseudohypoxia; increased expression of peroxisome proliferator-activated receptor alpha (PPARα), which induces fatty acid utilization and oxidation; increased fatty acid oxidation leading to the production of acetyl-CoA; increased production of advanced glycation end products (AGE), which leads to the irreversible modification of proteins; induction of the carbohydrate response element binding protein (ChREBP); and increased ROS production. The production of ROS is induced by oxidative stress due to the increased ratio of NADH/NAD+, and by AGE via signaling through its receptor (RAGE). ROS, ChREBP, and pseudohypoxia induce transcription of Hif1a mRNA. However, in the diabetic environment, HIF-1 signaling is negatively affected via modification of its coactivator p300, and by HIF-1α protein modifications.