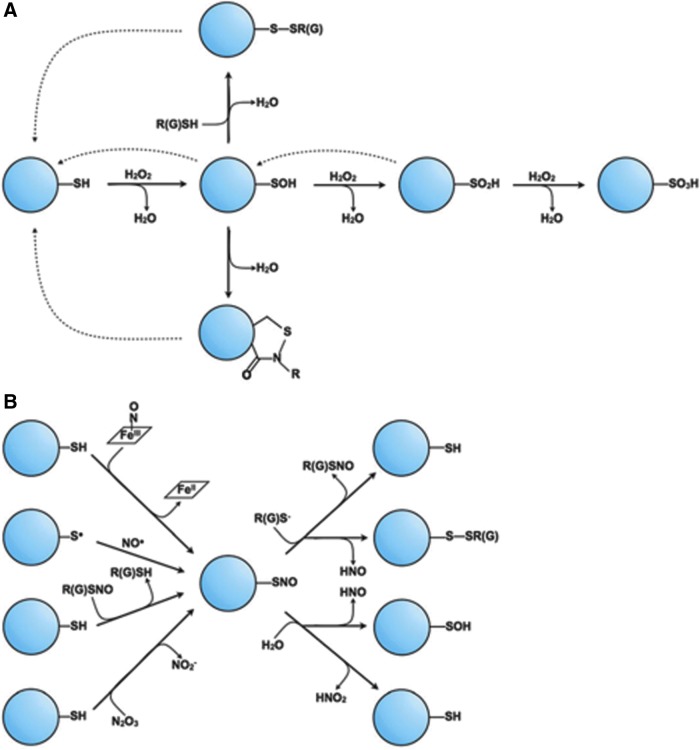
FIG. 1.
The range of oxidative/nitrosative cysteine post-translational modifications. (A) Oxidative modifications of cysteine can be characterized by its sequential redox reactions with H2O2 beginning with free thiolate. As additional equivalents of H2O2 react with cysteine, higher-order oxidations occur, from sulfenic acid (-SOH), to sulfinic acid (-SO2H), and finally sulfonic acid (-SO3H). Both sulfenic and sulfinic acids can be reduced through enzymatic reactions (dashed arrows). Sulfenic acids can also undergo further reactions with peptide backbone nitrogens to form sulfenamide adducts or with other protein or small-molecule thiols to form disulfides, which are also enzymatically reversible. (B) Nitrosative modifications occur through a number of distinct mechanisms that all involve NO or its derivatives (such as ONOO−). The fate of this SNO modification depends on the relative reactivity of the nitrogen and sulfur electrophiles; reaction with water can lead to either a sulfenic acid or a free thiolate, whereas reaction with a free thiol can result in either disulfide formation or transnitrosation. GSH, glutathione; H2O2, hydrogen peroxide; NO, nitric oxide; ONOO−, peroxynitrite; SNO, S-nitrosothiol. [Reproduced with some modifications with permission from Bak and Weerapana (7).]