Figure 3.
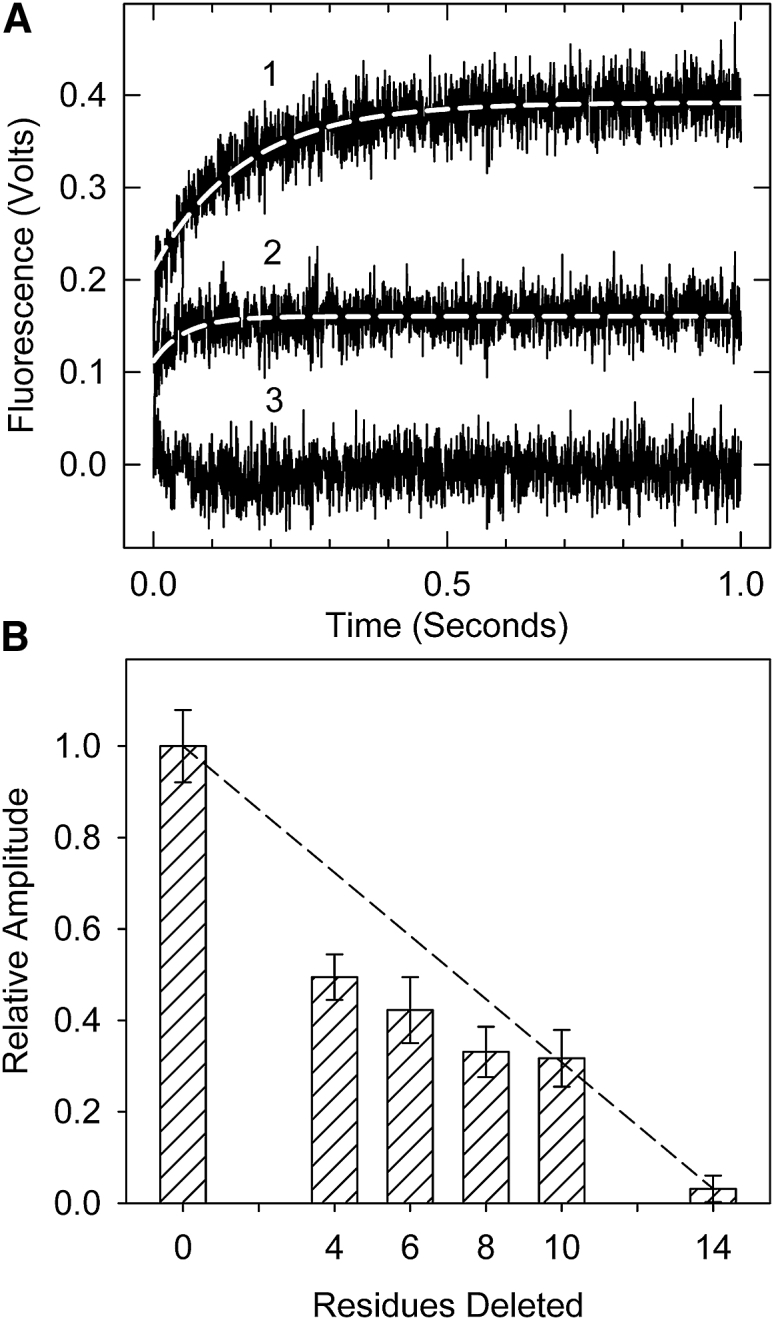
Formation of the inactive B-state seen by acrylodan-tropomyosin fluorescence. (A) Time courses of acrylodan fluorescence changes are shown after the rapid detachment of myosin S1 in the absence of Ca2+ at 10°C. Traces shown are averages of at least three different measurements. (B) Relative fluorescence amplitude for actin filaments containing wild-type and truncated troponin T is shown. The associated apparent rate constants were 6.8, 6.9, 6.5, 7.4, and 6.1 per s for the wild-type through Δ10 mutants, respectively. No rate constant could be determined for Δ14. Conditions are as follows: 2 μM actin, 0.86 μM tropomyosin, 1.4 μM troponin, and 2 μM S1 in 20 mM MOPS, 152 mM KCl, 4 mM MgCl2, 1 mM dithiothreitol, and 2 mM EGTA were rapidly mixed with 2 mM ATP, 20 mM MOPS, 152 mM KCl, 8 mM MgCl2, 1 mM dithiothreitol, and 2 mM EGTA. The dashed line shows the expected behavior if each residue contributed equally to the effect measured.
