Abstract
Perivascular stem cells (PSC) are a progenitor population defined by their perivascular residence. Recent studies have examined the relative difference in Wnt ligands to induce PSC differentiation, including Wnt16. Here, we examine the role of Wnt16 in the proliferation and osteogenic differentiation of human PSC. Treatment of PSC with WNT16 significantly increased cell proliferation to a greater extent than did WNT3A. In addition, WNT16 showed a significant increase in osteogenic gene expression among PSC. These data demonstrate that WNT16 represents a combined mitogenic/pro-osteogenic stimulus that may play a functional role in human mesenchymal stem cell mediated bone repair.
Keywords: Perivascular stem cell, PSC, Wnt signaling, Osteogenesis, WNT3A, DKK1
1. Introduction
Mesenchymal stromal cells (MSC) are a multipotent stromal cell population capable of mesenchymal differentiation into multiple cell types, including: adipogenic, chondrogenic, and osteogenic cell fates.1 Multiple applications exist for MSC in tissue regeneration, which primarily lie in MSC ability to function as paracrine regulators of tissue repair.2 Adipose tissue (AT) is an appealing cell source as it is readily available, accessible and dispensable by routine liposuction procedures. The stromal vascular fraction (SVF) has been previously used for bone repair, but forms bone tissue unreliably3 or with a low efficacy.4
To improve upon existing AT stromal therapies, we previously purified a population of MSC termed perivascular stem/stromal cells (PSC) from the SVF of human subcutaneous white adipose tissue.5 PSC are purified by fluorescence activated cell sorting (FACS) and represent a comparatively homogenous MSC population for regenerative medicine applications.6,7 PSC are abundant in human white adipose tissue, and are present in clinically relevant numbers for efforts in tissue engineering (∼40% of viable mononuclear SVF).7 PSC originate in the vessel wall, which represents a well-established source of mesenchymal progenitor cells.8,9 PSC are composed of two distinct yet related cell populations, including pericytes (CD34-CD146 + CD45-) and adventitial progenitor cells (CD34 +CD146-CD45-).9,10 PSC have been shown to promote in vivo bone regeneration across animal models, including a rat spinal fusion model6,11 and a calvarial defect model.12
The commitment of MSC to an osteogenic cell fate relies on many signaling, including both β-Catenin dependent canonical Wnt signaling, and β-catenin independent noncanonical Wnt signaling.13, 14, 15 See16 for a review of canonical and non-canonical Wnt signal transduction. WNT16, a mixed canonical and non-canonical Wnt signaling ligand, was previously observed to be enriched within the transcriptome of human PSC. In our recent observations,17 we found that sustained treatment with rWNT16 increased osteogenic differentiation in a c-Jun N-terminal kinase (JNK) pathway dependent fashion. In contrast, sustained rWNT3A treatment significantly decreased PSC osteogenic differentiation. Conversely, WNT16 knockdown significantly diminished PSC osteogenic differentiation. These data suggested that WNT16 plays a functional and necessary role in PSC osteogenesis. Here, we examine in more detail the role of recombinant Wnt16 in the proliferation and osteogenic differentiation of human PSC in vitro.
2. Methods
2.1. Perivascular stem/stromal (PSC) cell isolation
PSC were isolated from human lipoaspirate via fluorescence activated cell sorting (FACS). The stromal vascular fraction (SVF) was obtained by collagenase digestion. Briefly, lipoaspirate was diluted with an equal volume of phosphate-buffered saline (PBS) before digestion with Dulbecco's modified Eagle's medium (DMEM) containing 3.5% bovine serum albumin (Sigma-Aldrich, St. Louis) and 1 mg/ml type II collagenase for 70 min under agitation at 37 °C. Next, adipocytes were separated and removed by centrifugation. The pellet was then resuspended in red-cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA) and incubated for 10 min at room temperature. After centrifugation, pellets were resuspended in PBS and filtered at 70 μm. The resulting SVF was incubated with a mixture of the following directly conjugated antibodies: anti-CD34-phycoerythrin (1:100; Dako, Glostrup, Denmark), anti-CD45-allophycocyanin (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA), and anti-CD146-fluorescein isothiocyanate (1:100; AbD Serotec, Raleigh, NC). All incubations were performed at 4 °C for 15 min in the dark. Before sorting, 4′,6-diamidino-2-phenylindole (DAPI; 1:1000; Invitrogen, Carlsbad, CA) was added for dead cell exclusion; the solution was then passed through a 70-μm cell filter and then run on a FACSAria cell sorter (BD Biosciences, San Diego, CA). Sorted cells were plated for in vitro studies. In this manner, pericytes (CD34−CD146 + CD45−) and adventitial cells (CD34 +CD146−CD45−) were isolated and combined to constitute the PSC population. Cells were cultured at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. The expansion of cells was performed in DMEM, 20% fetal bovine serum (FBS), 1% penicillin/streptomycin. Medium was changed every 3 d unless otherwise noted.
2.2. Cell proliferation
PSC were seeded in 96 well plates at a density of 1000 cells per well and allowed to adhere overnight. Cells were cultured in DMEM + 20% FBS + 1% Pen Strep and treated with rWNT16 (80 ng/mL), rWNT3A (50 ng/mL), or rDKK1 (50 ng/mL) for 3 d followed by MTS assay per the manufacturer's instructions (Promega, Madison, WI).
2.3. Osteogenic differentiation
Assays for PSC differentiation are adapted from our prior publications.18,19 The osteogenic differentiation medium (ODM) included 10 mM β-glycerophosphate and 50 μM ascorbic acid in DMEM + 20% FBS. ODM with recombinant proteins was changed every third d. RNA isolation for specific gene expression was performed on 0, 3, 6, and 9 d of differentiation.
2.4. Ribonucleic acid (RNA) isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Gene expression was assayed by quantitative RT-PCR, based on our previous methods.19,20 Primers are in Supplemental Table 1. Timepoints for specific gene expression include 0, 3, 6, and 9 d of differentiation. Briefly, total RNA was extracted using RNEasy Kit (Qiagen, Santa Clarita, CA). 1 μg of total RNA from each sample was subjected to first-strand complementary deoxyribonucleic acid (cDNA) synthesis using the SuperScript III Reverse-Transcriptase Kit (Life Technologies) to a final volume of 20 μL The reverse transcription reaction was performed at 65 °C for 5 min, followed by 50 °C for 50 min and 85 °C for 5 min. For qRT-PCR, the reaction was performed using 2 × SYBR green RT-PCR master mix and an ABI PRISM 7300 qRT-PCR system instrument (Applied Biosystems, Foster City, CA). qRT-PCR was performed using 96 well optical plates at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and at 60 °C for 60 s. The relative quantification of gene expression was performed using a Comparative CT method according to the manufacturer's protocol and was normalized to the expression levels of the housekeeping gene, ACTB, in each sample.
2.5. Statistical analysis
All results were expressed as mean ± standard deviation (SD). Statistical analyses were performed using the SPSS16.0 software. All data were normally distributed. Student's t-test was used for two-group comparisons, and one-way ANOVA test was used for comparisons of 3 or more groups, followed by Tukey's post hoc test. Differences were considered significant when P < 0.05.
3. Results
3.1. Perivascular stem/stromal derivation
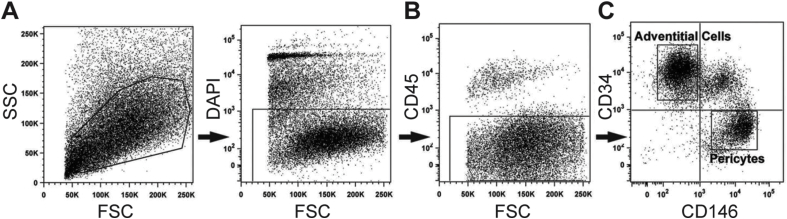
First, perivascular stem/stromal cells (PSC) were purified from human lipoaspirate using FACS to detect a population of pericytes and adventitial progenitor cells based on expression of CD146 and CD34 (Fig. 1). Briefly, using previously established protocols, the stromal vascular fraction (SVF) of lipoaspirate was processed so as to remove DAPI + non-viable cells (Fig. 1A), as well as CD45 + hematopoietic cells (Fig. 1B). Next, pericytes were defined as a CD146 + CD34-CD45-cell population while adventitial progenitor cells are CD34 +CD146-CD45-cell population (Fig. 1C). When combined, this bipartite population is termed PSC.9,10 Prior studies have confirmed that PSC have multilineage differentiation potential, including an ability to differentiate down osteogenic, adipogenic and chondrogenic lineages.9,10
Fig. 1.
Human perivascular stem/stromal cell (hPSC) isolation and osteogenic differentiation. (A–C) Fluorescence-activated cell sorting (FACS) isolation method for hPSC. (A) DAPI + non-viable cells and (B) CD45 + hematopoietic cells were excluded from the stromal vascular fraction of human lipoaspirate. (C) Purified hPSC consist of CD146 + CD34-pericytes and CD34 +CD146-adventitial cells. Reproduced with permission from Askarinam & James et al., Tissue Eng Part A, 2013, PMCID: PMC3638559.
3.2. WNT16 induces perivascular stem cell proliferation
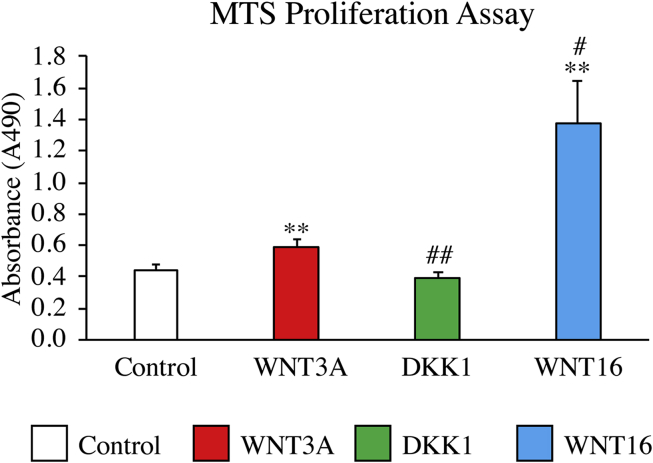
Our prior examination identified an enrichment of WNT16 transcripts among human PSC, and that WNT16 represented a context dependent stimulator of osteogenic and adipogenic differentiation.17 As well, WNT16 gene expression was observed to peak early in the process of PSC osteogenic differentiation (day 3), suggesting a role in osteoprogenitor cell expansion or early commitment.17 We next sought to examine the effects of WNT16 on cellular proliferation in more detail among hPSC. Cells were exposed to recombinant WNT16 (80 ng/mL) or the canonical ligand WNT3A (Fig. 2). As a further control, cells were also incubated with DKK1 (50 ng/mL). Results showed that at 3 days of osteogenic differentiation, WNT16 treated cells proliferated at ∼3 times the rate of the control, whereas the cells treated by WNT3A proliferated only slightly more rapidly (32% rate increase). In contrast, the DKK1 treated cells showed no significant change in proliferation rate. In summary, WNT16 significantly increased cell proliferation, and did so to a greater extent than did WNT3A in human PSC (see Fig. 3).
Fig. 2.
The effects of canonical and noncanonical Wnt signaling on PSC proliferation. PSC proliferation was assessed by MTS assay, performed at 3 days. PSC were exposed to WNT3A (50 ng/mL), DKK1 (50 ng/mL), or WNT16 (80 ng/mL). *p < 0.05, **p < 0.01 versus control. #p < 0.05, ##p < 0.01 versus WNT3A.
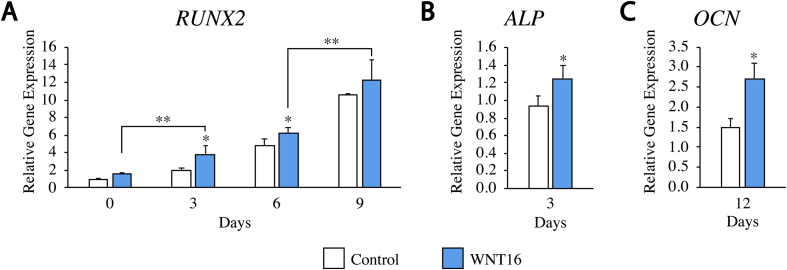
Fig. 3.
The effects of WNT16 on osteogenic gene expression. PSC were cultured in osteogenic differentiation medium (10 mM β-glycerophosphate and 50 μM ascorbic acid in DMEM + 20% FBS) with or without exogenous WNT16 (80 ng/mL). Gene expression of RUNX2 (0,3,6, and 9 days), ALP (3 days), and OCN (12 days) of differentiation. *p < 0.05, **p < 0.01 versus control.
3.3. WNT16 treatment enhances osteoblastogenic gene expression
Next, PSC were next exposed to exogenous WNT16 under osteogenic differentiation conditions. Previously, we found that sustained WNT16 treatment significantly increased osteogenic differentiation in a JNK signaling-dependent manner.17 Here, we inquired as to whether changes in cell proliferation were accompanied by changes in osteoblastogenic gene expression among human PSC in vitro. Results showed that markers of osteogenic differentiation, ALP, RUNX2, and OCN were significantly elevated in the rWNT16 treated group in comparison to the control group from the same day. Specifically, on days 3, 6, and 9, RUNX2 was increased. While the RUNX2 increase peaked on day 9 (75.5% increase over control), ALP increase was measured (74.9% increase over control) on day 3. OCN, a late marker of osteogenesis, was elevated at day 12 after osteogenic induction (55.7% increase over control). In summary, WNT16 represents a combined mitogenic/pro-osteogenic stimulus in human PSC in vitro.
4. Discussion
Our findings reinforce the importance of mixed canonical and noncanonical Wnt ligands, such as WNT16, in the process of osteogenic differentiation of human PSC. Overall, this lies in contrast to canonical Wnt ligands such as WNT3A, which tends to inhibit MSC osteogenesis when applied in a sustained fashion.21,22 WNT16 is generally recognized as a mixed canonical and noncanonical Wnt signaling ligand in osteoblastic cells.23 Although it has been hypothesized that WNT16 mainly stimulates bone formation through canonical signaling, while inhibiting osteoclast formation through non-canonical signaling,23 there have also been evidence supporting WNT16's ability to effect bone formation via non-canonical signaling.24 Indeed in human PSC, WNT16's ability to stimulate noncanonical activity via the JNK pathway was required for osteogenic differentiation.17 Knockdown of WNT16 has inhibited MSC osteoblastogenesis, either in human skeletal muscle MSC25 or in AT derived PSC.17
Overall, an purified AT derived stromal cell therapy has significant advantages over currently available bone graft substitute products. The gold standard for regeneration is autograft bone.26,27 However, bone grafts are encumbered by numerous disadvantages, including donor site morbidity,26,28 complications of extended operating time, and limitations in autogenous supply. Alternatives to autograft bone are numerous, however each has significant drawbacks.29,30 Demineralized bone matrix is processed to reduce immunogenicity, but this processing also eliminates stem cells in comparison to autograft bone. Disadvantages are also present with the commonly used growth factor, Bone Morphogenetic Protein (BMP)-2), confirmed by independent reviews in the Yale University Open Data Access project.31, 32, 33 Adverse effects include osteoclast activation,34 life-threatening inflammatory swelling,35 and inappropriate adipogenesis.36, 37, 38 Overall the combined mitogenic/pro-osteogenic effects of WNT16 suggest the utility of a combined growth factor + autologous cell therapy approach for bone tissue engineering.
Conflict of Interest
None.
Author disclosure statement
None.
Acknowledgments
The present work was supported by the NIH/NIAMS (R01 AR070773, K08 AR068316), NIH/NIDCR (R21 DE027922), Department of Defense (W81XWH-18-1-0121, W81XWH-18-1-0336), American Cancer Society (Research Scholar Grant, RSG-18-027-01-CSM), the Maryland Stem Cell Research Foundation, and the Musculoskeletal Transplant Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or Department of Defense.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jor.2018.08.021.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cell. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 2.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller A.M., Mehrkens A., Schäfer D.J. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–135. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 4.Cheung W.K., Working D.M., Galuppo L.D., Leach J.K. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12:554–562. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 5.Chung C.G., James A.W., Asatrian G. Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med. 2014;3:1231–1241. doi: 10.5966/sctm.2014-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James A.W., Zara J.N., Corselli M. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1:673–684. doi: 10.5966/sctm.2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James A.W., Zara J.N., Zhang X. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray I.R., West C.C., Hardy W.R. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crisan M., Yap S., Casteilla L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Corselli M., Chen C.W., Sun B., Yap S., Rubin J.P., Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cell Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askarinam A., James A.W., Zara J.N. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng. 2013;19:1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James A.W., Zara J.N., Corselli M. Use of human perivascular stem cells for bone regeneration. JoVE. 2012:e2952. doi: 10.3791/2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Alimonte I., Lannutti A., Pipino C. Wnt signaling behaves as a "master regulator" in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013;9:642–654. doi: 10.1007/s12015-013-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Boer J., Wang H.J., Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 15.Königshoff M., Kramer M., Balsara N. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravenall S.J., Gavazzi I., Wood J.N., Akopian A.N. A peripheral nervous system actin-binding protein regulates neurite outgrowth. Eur J Neurosci. 2002;15:281–290. doi: 10.1046/j.0953-816x.2001.01862.x. [DOI] [PubMed] [Google Scholar]
- 17.Shen J., Chen X., Jia H. Effects of WNT3A and WNT16 on the osteogenic and adipogenic differentiation of perivascular stem/stromal cells. Tissue Eng. 2018 Jan;24(1-2):68–80. doi: 10.1089/ten.tea.2016.0387. Epub 2017 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S., Zhang X., Shen J. Brief report: human perivascular stem cells and nel-like Protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cell. 2015;33:3158–3163. doi: 10.1002/stem.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James A.W., Shen J., Zhang X. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen J., James A.W., Zhang X. Novel Wnt regulator NEL-like Molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186:419–434. doi: 10.1016/j.ajpath.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belinsky G.S., Sreekumar B., Andrejecsk J.W. Pigment epithelium-derived factor restoration increases bone mass and improves bone plasticity in a model of osteogenesis imperfecta type VI via Wnt3a blockade. Faseb J. 2016;30:2837–2848. doi: 10.1096/fj.201500027R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boland G.M., Perkins G., Hall D.J., Tuan R.S. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 23.Movérare-Skrtic S., Henning P., Liu X. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20:1279–1288. doi: 10.1038/nm.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrickx G., Boudin E., Fijałkowski I. Variation in the Kozak sequence of WNT16 results in an increased translation and is associated with osteoporosis related parameters. Bone. 2014;59:57–65. doi: 10.1016/j.bone.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Ozeki N., Mogi M., Hase N. Bone morphogenetic protein-induced cell differentiation involves Atg7 and Wnt16 sequentially in human stem cell-derived osteoblastic cells. Exp Cell Res. 2016;347:24–41. doi: 10.1016/j.yexcr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Laurencin C., Khan Y. Bone graft substitute materials. Emedicine. 2004. http://www.emedicine.com/orthoped/topic611.htm
- 27.Bauer T.W., Muschler G.F. Bone graft materials. An overview of the basic science. Clin Orthop. 2000 Feb;(371):10–27. 2000:10-27. [PubMed] [Google Scholar]
- 28.Frodel J.L., Jr., Marentette L.J., Quatela V.C., Weinstein G.S. Calvarial bone graft harvest. Techniques, considerations, and morbidity. Arch Otolaryngol Head Neck Surg. 1993;119:17–23. doi: 10.1001/archotol.1993.01880130019002. [DOI] [PubMed] [Google Scholar]
- 29.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Gazdag A.R., Lane J.M., Glaser D., Forster R.A. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3:1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Laine C., Guallar E., Mulrow C. Closing in on the truth about recombinant human bone morphogenetic protein-2: evidence synthesis, data sharing, peer review, and reproducible research. Ann Intern Med. 2013;158:916–918. doi: 10.7326/0003-4819-158-12-201306180-00012. [DOI] [PubMed] [Google Scholar]
- 32.Fu R., Selph S., McDonagh M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158:890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 33.Simmonds M.C., Brown J.V., Heirs M.K. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158:877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 34.Irie K., Alpaslan C., Takahashi K. Osteoclast differentiation in ectopic bone formation induced by recombinant human bone morphogenetic protein 2 (rhBMP-2) J Bone Miner Metabol. 2003;21:363–369. doi: 10.1007/s00774-003-0430-x. [DOI] [PubMed] [Google Scholar]
- 35.Schultz D. FDA Public Health Notification: Life-threatening Complications Associated With Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. in: Health CfDaR, ed2008.
- 36.Kang Q., Song W.X., Luo Q. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cell Dev. 2009;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sottile V., Seuwen K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett. 2000;475:201–204. doi: 10.1016/s0014-5793(00)01655-0. [DOI] [PubMed] [Google Scholar]
- 38.Hata K., Nishimura R., Ikeda F. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell. 2003;14:545–555. doi: 10.1091/mbc.E02-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.