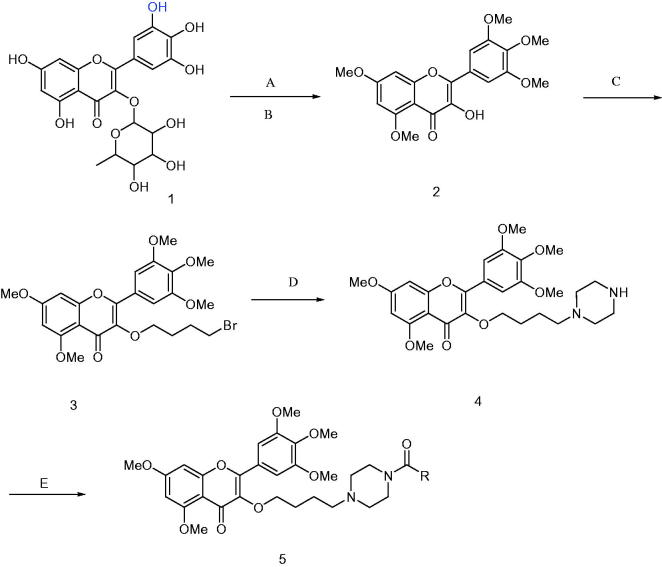
Scheme 1.
Synthesis of compounds 5a∼5l. 5a: R = 2,4-difluorobenzoyl 5b: R = 2,6-difluorobenzoyl 5c: R = 2-chloro-6-fluorobenzoyl 5d: R = 3-fluorobenzoyl 5e: R = 2-nitrobenzoyl 5f: R = 4-trifluoromethyl 5g: R = 4-nitrobenzoyl 5h: R = 4-chlorobenzoyl 5i: R = isonicotinoyl 5j: R = benzoyl 5k: R = 3,5-dinitrobenzoyl 5l: R = 5-chloro-2-nitrobenzoyl. Reagent and conditions: (A) K2CO3, DMF, CH3I, r.t. 48 h; (B) 5% HCl, 95% ethanol, reflux, 2 h; (C) Br(CH2)4Br, acetone, K2CO3, reflux, 12 h; (D) K2CO3, DMF, Piperazine, r.t. 12 h; (E) HATU, DCM, RCOOH, r.t. 12 h.