Figure 1:
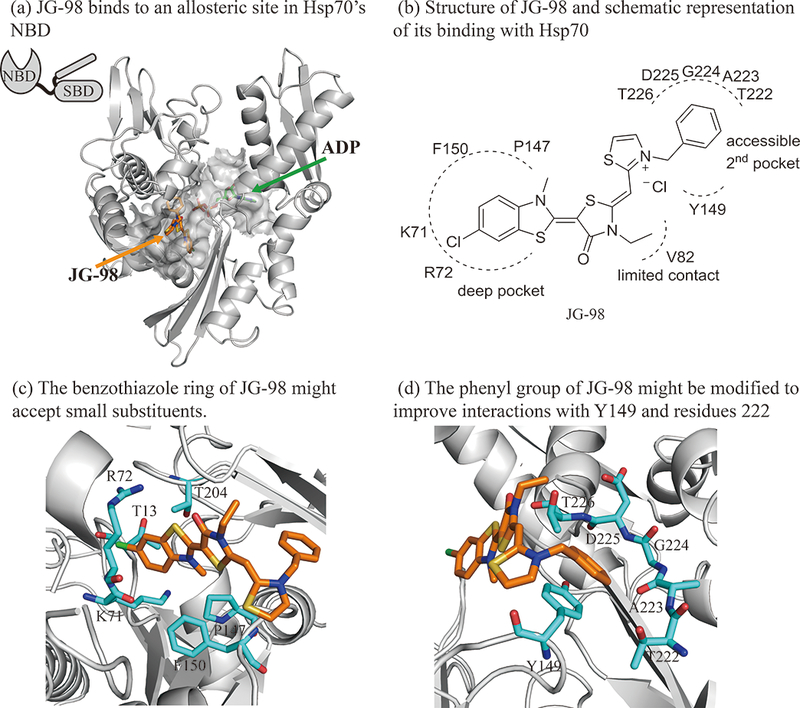
Structure guided design of Hsp70 inhibitor JG-98 analogs. (a) JG-98 binds to an allosteric site in the nucleotide binding domain (NBD) of Hsp70, rather than its necleotide binding site. JG-98 was docked into HSPA8 using flexible-receptor flexible-ligand (InducedFit) docking. The carbon atoms of the protein, JG-98 and ADP are shown as gray, orange and green, respectively. JG-98 and ADP are shown as stick representations. (b) Schematic representation of JG-98 interacting with Hsp70. (c) The benzothiaole ring of JG-98 is located in a deep hydrophobic pocket formed by R72, K71, F150, P147, T204 and T13. (d) The phenyl ring forms favorable interactions with Y149 and the backbone atoms of residues 223 through 226. Selected residues are shown in cyan as stick representation.
