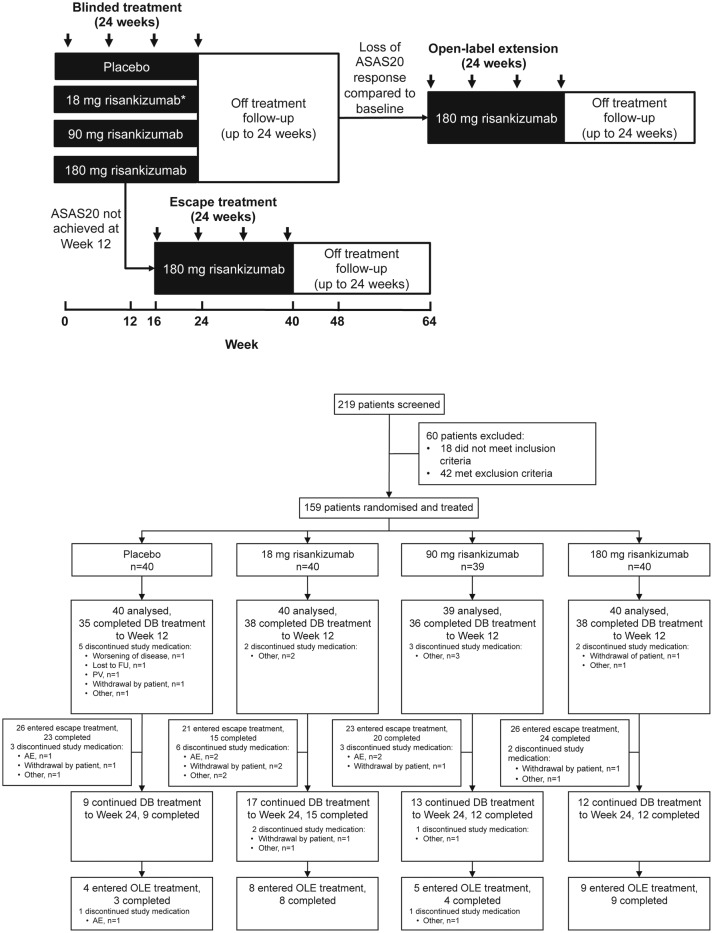
Figure 1.
Overview of study design and patient disposition. Overview of treatment and observation periods including escape and open-label extension phases (panel A); patients were randomised 1:1:1:1 to one of three regimens of risankizumab (18 mg single dose, 90 mg or 180 mg at day 1 and weeks 8, 16 and 24) or placebo; patients without ASAS20 response at week 12 received escape treatment; patients with a flare of disease activity within 24 weeks of the last double-blind treatment entered the open-label extension. Arrows represent treatment administration. *Patients received 18 mg single dose at day 1 followed by placebo at weeks 8, 16 and 24. Trial profile (panel B). AE, adverse event; ASAS20, 20% improvement in Assessment in SpondyloArthritis International Society; DB, double blind; FU, follow-up; OLE, open-label extension; PV, protocol violation.