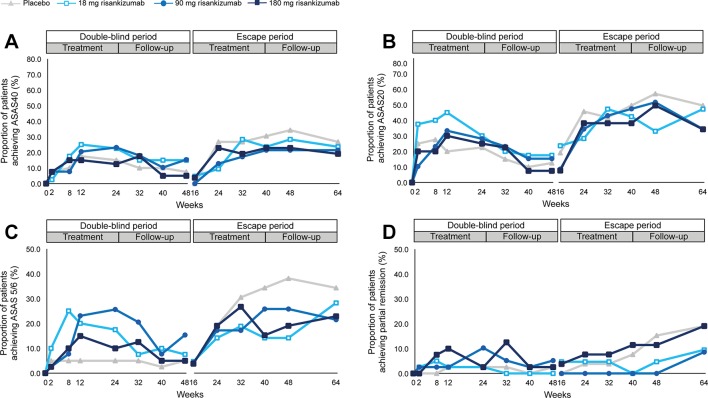
Figure 2.
Response rates for ASAS40, ASAS20, ASAS 5/6 and ASAS partial remission during double-blind and escape treatment and follow-up periods. Clinical response rates over time for double-blind and escape treatment periods. ASAS40 (panel A), ASAS20 (panel B), ASAS 5/6 (panel C) and partial remission (panel D). NRI was used for missing data. Number of patients entering the double-blind treatment were: placebo: n=40; 18 mg risankizumab: n=40; 90 mg risankizumab: n=39; and 180 mg risankizumab: n=40. Patients entering escape treatment received 180 mg risankizumab; responses shown for the escape period are by the original randomised treatment (placebo: n=26; 18 mg risankizumab: n=21; 90 mg risankizumab: n=23; 180 mg risankizumab: n=26). Values for all data points are provided in online supplementary tables S1–S4. ASAS, Assessment in SpondyloArthritis International Society; NRI, non-responder imputation.