Abstract
Background
Genotype 3 (GT3) is a common chronic hepatitis C (CHC) genotype in Asia. Direct-acting antiviral (DAA) regimens have high cure rates, but real-world results are limited for Asia.
Aim
To determine the real-world effectiveness of DAAs for patients with CHC GT3 in Asia.
Methods
A systematic search was performed in PubMed (including MEDLINE), Embase, and selected international meeting abstract repositories. Eligible studies were postmarketing observational studies from Asia with the primary outcome of sustained virological response 12 weeks after completion of treatment (SVR12).
Results
A total of 15 studies with 4230 patients yielded a pooled SVR12 of 92.7%. High heterogeneity (I2=93.2%, P<0.0001) was noted. In subgroup analyses, patients with cirrhosis had 10.9% lower SVR12 than non-cirrhotic patients (88.6% vs 98.9%; P<0.0001) and contributed 69.5% of the heterogeneity. Prior treatment failure did not reduce the pooled SVR12 (treatment-naïve: 94.6%, 95% CI 91.3% to 96.7% vs treatment-experienced: 94.0%, 95% CI 77.5% to 98.6%; P=0.89). Twenty-four weeks of sofosbuvir+ribavirin dual therapy was the most commonly used regimen which led to similar SVR12 (OR=1.1, P=0.73) but lower adverse event rate than 12 weeks of sofosbuvir+ribavirin+pegylated interferon triple therapy.
Conclusion
Sofosbuvir+ribavirin for 24 weeks is the most widely used and generally well-tolerated DAA therapy in Asia. However, its effectiveness is not optimal in GT3 patients with cirrhosis.
Keywords: hepatitis C, liver cirrhosis, genotype, adverse drug reactions
Introduction
Hepatitis C virus (HCV) infection is a significant health burden around the world affecting an estimated 1% of the global population (71.1 million HCV infections).1 HCV genotype 3 (GT3) accounts for 25% of the total HCV cases. It is the second most common genotype in Asia overall and the most common HCV genotype in certain parts of Asia, representing 66.7%, 19.9%, 19.6%, 5.4% and 0.4% among all HCV genotypes in South Asia, South-East Asia, Central Asia, East Asia, and high-income Asia Pacific countries, respectively.1 2 Since the new direct-acting antiviral (DAA) therapies were launched in late 2013, the sustained virological response (SVR) is now nearly 100% among many HCV patient groups with most genotypes and patient subgroups in clinical trials. However, GT3 is associated with lower SVR rates than other genotypes.3 Despite the large number of HCV GT3 infections in Asia, pivotal clinical trials of DAAs in patients with HCV GT3 included very few Asians. Specifically, only 9% of the patients enrolled in major registration trials on the use of DAAs for treatment of patients with HCV GT3 were Asian, which included only 9 and 7 among the total of 250 and 152 patients in the VALENCE and ALLY-3 clinical trials, respectively, and 80 patients among the 592 GT2 and GT3 patients in the BOSON clinical trial (online supplementary table S1).4–7
bmjgast-2018-000209supp001.pdf (322.4KB, pdf)
Daclatasvir (DCV) + asunaprevir, ledipasvir (LDV)/sofosbuvir (SOF) and SOF + ribavirin (RBV) combination therapies became available to Asia in 2014/2015.8–12 SOF was approved in India in March 2015, while SOF+RBV treatment regimen was approved in Pakistan in November 2014.9–12 Based on the Asian Pacific Association for the Study of Liver Disease (APASL) 2016 guidelines, recommended therapies for HCV GT3 include (1) SOF+weight-based RBV for 24 weeks for treatment-naïve patients; (2) SOF+DCV for 12 weeks for those without cirrhosis, and SOF+DCV for 24 weeks with or without weight-based RBV for those with cirrhosis; and (3) among patients who are treatment-experienced with pegylated interferon (Peg-IFN), SOF+DCV for 12 weeks was recommended for non-cirrhotic patients and SOF+DCV with weight-based RBV for 24 weeks for patients with cirrhosis.13 The current Indian National Association for Study of the Liver recommends (1) SOF+DCV for 12 weeks or SOF+weight-based RBV for 24 weeks for those unwilling/intolerant to DCV non-cirrhotic patients; (2) SOF+DCV+weight-based RBV for 24 weeks for patients with cirrhosis, and alternative SOF+weight-based RBV+Peg-IFN for 12 weeks for patients with compensated cirrhosis or SOF+RBV for up to 48 weeks for patients with decompensated cirrhosis.10 In 2017, the Pakistan National Consensus Practice Guidelines of Hepatitis C offered the three following recommendations for GT3 patients: (1) SOF+DCV for 12 weeks or SOF+velpatasvir (VEL) for 12 weeks for both treatment-naïve and IFN/RBV treatment-experienced and (2) SOF+weight-based RBV+Peg-IFN for 12 weeks for IFN-eligible patient or SOF+weight-based RBV for 24 weeks for IFN-ineligible patient; and (3) SOF+DCV for 24 weeks or SOF+VEL for 12 weeks for either non-cirrhotic patients or patients with cirrhosis.11
However, since the introduction of the new DAAs to Asia, real-world effectiveness results have been sporadically published. Therefore, to gain a real-world perspective of the effectiveness of the new DAA regimens among Asian patients with HCV GT3, we performed a systematic review and meta-analysis of the current literature on the treatment of HCV GT3 from postmarketing studies conducted in Asia.
Materials and methods
Data sources and search strategy
Our study protocol was registered in PROSPERO (CRD: 42017067928). We performed a systematic search of PubMed (including MEDLINE), Embase, and major international meeting abstracts from Digestive Disease Week, Asian Pacific Digestive Week, the American Association for the Study of Liver Diseases, the European Association for the Study of the Liver, and APASL until 30 June 2017. Search terms included ledipasvir, sofosbuvir, simeprevir, daclatasvir, asunaprevir, ombitasvir, dasabuvir, HCV, and hepatitis C. The searches were further restricted to Asian countries (online supplementary list 1).14 There was no language restriction. We defined the real-world evidence based on the definitions of Sherman and colleagues.15
Study selection
Eligible studies were non-clinical trial original studies in Asian real-world settings with the primary outcome of SVR12, and/or secondary outcome of tolerability information for adult patients (≥18 years) with chronic hepatitis C(CHC) GT3. We excluded phase I–III premarketing clinical trial studies and studies with small sample sizes of 10 patients or fewer.
Data extraction
A standardised case report form was designed for data extraction. The study information collected included authors’ name, authors’ organisation, publication year, study country, study period, study centre, sample size, study participants’ characteristics including age, gender distribution, subtypes for GT3, cirrhosis status, prior treatment status, primary outcomes of overall DAA SVR12, and secondary outcomes of subgroups’ effectiveness and tolerability, including the number and type of common adverse events (AEs) and serious adverse events. Two reviewers (BW and FJ) independently identified, screened, extracted, discussed, and evaluated eligible studies. A third researcher (MHN) resolved any discordance.
Statistical analyses
Meta-analyses were conducted for SVR12 in the overall study population and subgroups using the Freeman-Tukey double arcsine transformation and Wilson score CI in the random-effects model.16 For subgroup analysis, ORs were performed in studies with two therapeutic arms (SOF+RBV therapy with and without the addition of Peg-IFN), which involved weighted averages in random-effects models. We also calculated the pooled SVR12 within subgroups, including comparison of SVR12 by therapies, by cirrhosis status, by treatment history, by both cirrhosis status and treatment history, and by countries. The between-subgroup differences were tested with statistical level of significance defined with a P-value <0.05. In addition, meta-regression was performed for the statistically significant subgroups to quantify the magnitude of impact of subgroup effectors. R2 indices were used to quantify the impact of the subgroup effectors.17 Cochran Q test and I2 statistics were quantified for heterogeneity, with cut-offs of 25%, 50% and 75% to suggest low (25%-50%), moderate (50%-75%), and high (>75%), respectively.18 Egger’s test and Begg’s test were evaluated for potential publication bias.19 20
A sensitivity analysis was conducted for the pooled SVR12 results to check for accuracy affected by small sample sizes or low-quality studies.
All analyses were carried out in R V.3.3.2 (R Foundation for Statistical Computing, Vienna, Austria), with ‘meta’ and ‘ggplot2’ packages.21–23
We used the Newcastle-Ottawa Scale (NOS) to evaluate risk of bias.24 Studies were evaluated and discussed for discordance by two reviewers.
Results
Study characteristics
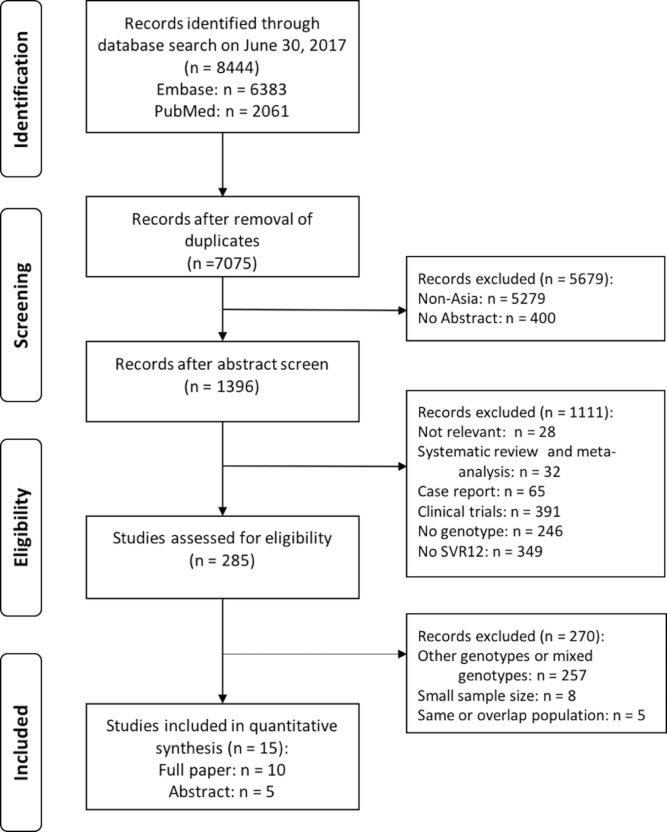
Table 1 and figure 1 show the results of the database search and the selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses format.25 Following the previously described search strategy, 15 studies were eligible for analysis, including 10 published as full articles and 5 as conference abstracts. Six studies were from India, six from Pakistan, two from Myanmar and one from Iran. Ten studies were single-centre, three were multicentre, and the study centre setting was not specified in two studies. Five studies were published in 2016 and ten in 2017.
Table 1.
Summary of included studies for systematic review and meta-analysis
| First author | Published year | Paper type | Country | Study centre | Patients (n) | Patients with LC (%) | DAA regimen |
| Abbas26 | 2017 | Abstract | Pakistan | Single-centre | 241 | 64 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Akhter27 | 2016 | Full paper | Pakistan | Single-centre | 55 | NA | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Capileno12 | 2017 | Full paper | Pakistan | Single-centre | 153 | 61 | SOF+RBV 24 weeks |
| Farooqi28 | 2016 | Abstract | Pakistan | NA | 47 | NA | SOF+RBV±Peg-IFN 12/24 weeks |
| Goel29 | 2017 | Full paper | India | Single-centre | 160 | 51 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks; DCV+SOF±RBV 12/24 weeks |
| Hlaing30 | 2017 | Full paper | Myanmar | Multicentre | 133 | 55 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks; DCV+SOF+RBV 12/24 weeks; LDV+SOF 12 weeks |
| Mehta31 | 2016 | Full paper | India | Single-centre | 67 | 64 | SOF+RBV±Peg-IFN 12/24 weeks |
| Merat32 | 2017 | Full paper | Iran | Single-centre | 44 | 100 | DCV+SOF+RBV 12 weeks |
| Sarwar33 | 2017 | Full paper | Pakistan | Single-centre | 198 | 52 | SOF+RBV±Peg-IFN 12/24 weeks |
| Satsangi34 | 2017 | Full paper | India | Single-centre | 105 | 33 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Shah35 | 2016 | Abstract | India | Multicentre | 59 | 24 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Sidhu36 | 2017 | Full paper | India | Multicentre | 931 | 21 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Sood9 | 2017 | Full paper | India | Single-centre | 589 | 46 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
| Win37 | 2016 | Abstract | Myanmar | NA | 73 | NA | SOF+RBV+Peg-IFN 12 weeks |
| Yousaf38 | 2017 | Abstract | Pakistan | Single-centre | 1375 | 0 | SOF+RBV 24 weeks; SOF+RBV+Peg-IFN 12 weeks |
DAA, direct-acting antiviral; DCV, daclatasvir; LC, liver cirrhosis; LDV, ledipasvir; NA, not available; Peg-IFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir.
Figure 1.
Screening of articles based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. SVR12, sustained virological response 12 weeks after completion of treatment.
Overall pooled SVR12 for DAA therapies in routine practice in Asia
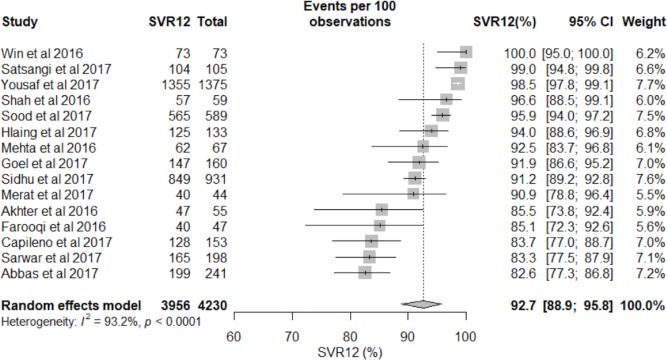
A total of 4230 GT3 patients were treated with DAAs and included in our final analysis.9 12 26–38The pooled SVR12 was 92.7% (95% CI 88.9% to 95.8%) (figure 2 and figure 3). There was high heterogeneity among the studies (I2=93.2%, P<0.0001), indicating that one or more subgroups may be the cause of the heterogeneity (see meta-regression analysis results below). Publication bias was not observed in either the Begg’s test (P=0.80) or Egger’s test (P=0.14).
Figure 2.
Overall sustained virological response 12 weeks after completion of treatment (SVR12) of direct-acting antiviral therapies for chronic hepatitis C genotype 3 in Asia.
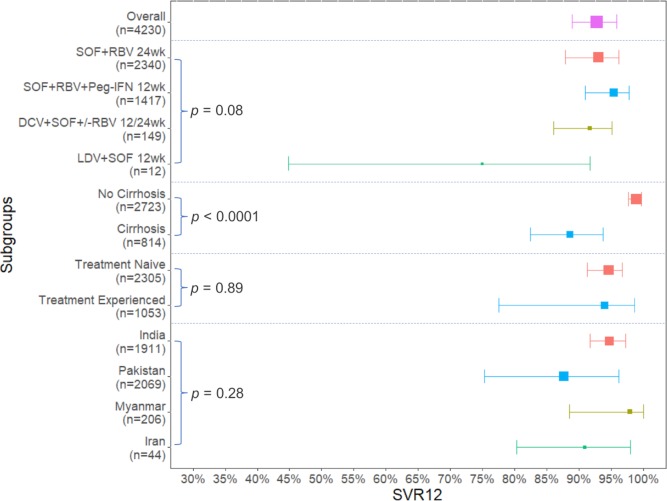
Figure 3.
Summary plots for overall and subgroup analyses in patients with chronic hepatitis C genotype 3 treated with direct-acting antiviral therapies in Asia. DCV, daclatasvir; LDV, ledipasvir; Peg-IFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir; SVR12, sustained virological response 12 weeks after completion of treatment.
Pooled SVR12 for individual DAA regimen
The majority of patients (n=4069, 96%) were treated with an SOF+RBV±Peg-IFN therapy: SOF+RBV for 24 weeks (n=2340) or SOF+RBV+Peg-IFN for 12 weeks (n=1417) or SOF+RBV±Peg-IFN for 12 or 24 weeks (n=312), with a pooled SVR12 of 93.0% (95% CI 87.9% to 96.1%, I2=89.3%), 95.4% (95% CI 90.9% to 97.7%, I2=81.9%), and 86.2% (95% CI 79.4% to 91.0%, I2=38.8%), respectively (online supplementary figure S1).9 12 26–31 33–38 In addition, DCV+SOF±RBV for 12 or 24 weeks of therapy was used in 149 patients with HCV GT3, with an overall SVR12 of 91.7% (95% CI 86.0% to 95.1%, I2=0%).29 30 32 The SVR12 of DCV+SOF±RBV was 96.0% (48/50) for non-cirrhotic patients and 89.1% (49/55) for patients with cirrhosis. Only 12 patients were treated with LDV+SOF for 12 weeks and 9 achieved SVR12 (75.0%).30 There was no statistically significant difference in the SVR12 rates among these four therapies (P=0.08) (figure 3 and online supplementary figure S1).
SVR12 for SOF+RBV for 24 weeks versus SOF+RBV+Peg-IFN for 12 weeks
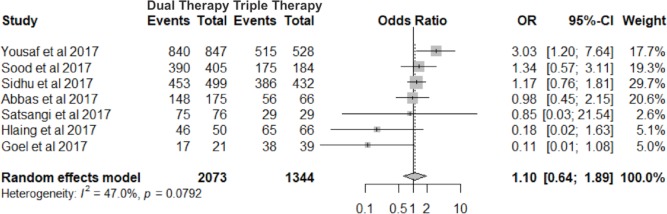
Seven of the 15 studies compared 24 weeks of SOF+RBV with 12 weeks of SOF+RBV+Peg-IFN for 12 weeks.9 26 29 30 34 38 In this subgroup analysis, we evaluated the ORs of SVR12 with a random-effects model between the two groups. There was no significant difference in SVR12 rates among patients treated with dual therapy versus triple therapy, OR=1.1 (95% CI 0.64 to 1.89, I2=47.0%), P=0.73 (figure 4).
Figure 4.
Forest plot for SVR12 subgroup comparison between 24 weeks of SOF+RBV and 12 weeks of SOF+RBV+Peg-IFN therapies. Peg-IFN, pegylated interferon; RBV, ribavirin; SOF, sofosbuvir; SVR12, sustained virological response 12 weeks after completion of treatment.
AEs for SOF+RBV for 24 weeks and SOF+RBV+Peg-IFN for 12 weeks
For patients treated with 24 weeks of SOF+RBV, AE data were available in 1346 patients from two studies.36 38 The most common AEs were anaemia (28.1%, 95% CI 25.2% to 31.2%), headache (23.5%, 95% CI 14.5% to 35.8%), fatigue (22.9%, 95% CI 13.1% to 37.1%), myalgia (11.0%, 95% CI 8.6% to 14.1%), and insomnia (7.8%, 95% CI 5.2% to 11.5%).
For patients treated with 12 weeks of SOF+RBV+Peg-IFN, AE data were available in 960 patients from two studies.36 38 The most common AEs were myalgia (49.1%, 95% CI 44.4% to 53.8%), fatigue (43.5%, 95% CI 21.6% to 66.8%), anaemia (40.7%, 95% CI 36.6% to 45.0%), and insomnia (12.0%, 95% CI 10.0% to 14.1%).
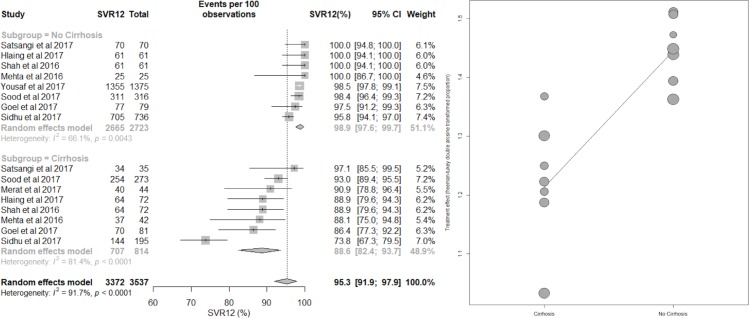
Meta-regression by cirrhosis status
There were significant differences in the pooled SVR12 between the cirrhotic and non-cirrhotic subgroups, with a statistically significantly lower response rate in cirrhotic subgroup. This analysis included 9 studies with 2723 non-cirrhotic patients and 814 patients with cirrhotic CHC GT3.9 29–32 34–36 38 The pooled estimate of SVR12 for non-cirrhotic and cirrhotic subgroups were 98.9% (95% CI 97.6% to 99.7%) and 88.6% (95% CI 82.4% to 93.7%), respectively (P<0.0001). Heterogeneity was reduced from 91.7% in total to I2=66.1% for the non-cirrhotic group and I2=81.4% for the cirrhotic group (figure 3 and figure 5). Meta-regression analysis also showed that patients with cirrhosis had a 10.9% (95% CI 5.4% to 16.1%) lower chance to achieve SVR12 than non-cirrhotic patients. The R2 index was 69.5%, indicating that cirrhosis may contribute 69.5% to the overall observed heterogeneity.
Figure 5.
Forest plot for sustained virological response 12 weeks after completion of treatment (SVR12) in patients with chronic hepatitis C genotype 3, by cirrhosis status.
Subgroup analysis by treatment history
A total of 3358 patients in 9 studies were eligible for the subgroup analysis based on treatment history.9 27 30–32 34–36 38 Without accounting for cirrhosis status, there were no significant differences in pooled SVR12 between treatment-naïve patients (pooled SVR12: 94.6%, 95% CI 91.3% to 96.7%) and patients with prior treatment failure (pooled SVR12: 94.0%, 95% CI 77.5% to 98.6%) (P=0.89) (figure 3 and online supplementary figure S2).
Subgroup analysis by treatment history and by cirrhosis status
We performed a stratified subgroup analysis by cirrhosis status and treatment history with 2581 DAA-treated patients with CHC GT3 in six studies.31 32 34–36 38 We found that non-cirrhotic treatment-naïve patients had the highest SVR12 at 98.0% (n=1324, 95% CI 96.6% to 98.8%, I2=17.2%). Non-cirrhotic treatment-experienced patients also had relatively high SVR12 at 91.1% (n=927, 95% CI 47.8% to 99.1%, I2=94.8%). As expected, patients with cirrhosis with prior treatment failure and cirrhotic treatment-naïve patients had lower pooled SVR12 at 88.2% (n=87, 95% CI 49.0% to 98.3%, I2=82.4%) and 83.1% (n=243, 95% CI 76.2% to 88.3%, I2=10.4%), respectively (online supplementary figure S3).
Subgroup analysis by country and meta-regression controlling for cirrhosis
There were 1911 patients treated with SOF+RBV±Peg-IFN or DCV+SOF±RBV in 6 studies from India, 2069 patients with SOF+RBV±Peg-IFN in 6 studies from Pakistan, 206 patients with SOF+RBV±Peg-IFN or DCV+SOF±RBV in 2 studies from Myanmar, and 44 patients treated with DCV+SOF±RBV in 1 study from Iran (table 1). The overall percentage of patients with cirrhosis among cohorts that included patients with cirrhosis was about 35% in India, 50% in Pakistan and 55% in Myanmar (table 1).
The pooled SVR12 estimates were 94.7% (95% CI 91.7% to 97.2%) for India, 87.6% (95% CI 75.3% to 96.2%) for Pakistan, 97.9% (95% CI 88.5% to 100.0%) for Myanmar, and 90.9% (95% CI 80.3% to 98.0%) for Iran without significant differences noted among the countries (P=0.28) (figure 3 and online supplementary figure S4).
Meta-regression controlling for the effect of cirrhosis showed no statistically significant difference in SVR12 rates among the countries, with regression coefficients of −0.06, 0.08 and 0.02 (P=0.06, 0.36 and 0.68) for Pakistan, Iran and Myanmar compared with India as the referent, respectively.
Quality assessment
We categorised a total score of 8 or higher as high-quality studies, 4–7 as fair-quality studies and 1–3 as low-quality studies. There were five high-quality, eight fair-quality and two low-quality studies included in our analysis. The average score in the NOS quality assessment was 6.3 out of a total score of 9 (online supplementary table S2).
Sensitivity analyses for heterogeneity
Sensitivity analyses excluding abstracts, studies with small sample size (<100) or studies with low-quality ratings did not result in significant changes in I2 or pooled SVR12 estimates (online supplementary table S3).
Discussion
Using a systematic review and meta-analysis approach to investigate real-world outcomes of DAA therapies in Asian patients with HCV GT3, we found an overall SVR12 of 92.7% from 15 studies with 4230 patients, which is a much higher SVR outcome than reported using the older Peg-IFN+RBV therapy.39 However, it should be noted that while non-cirrhotic patients experienced a very high SVR12 of 98.9%, SVR12 was only 88.6% for patients with cirrhosis. The majority of the patients included in our study were treated with either SOF+RBV for 24 weeks (n=2340) or SOF+RBV+Peg-IFN for 12 weeks (n=1417).
This real-world analysis for DAA therapies of GT3 patients in Asia interestingly showed a much higher SVR12 than results from the meta-analysis of clinical trials carried out globally for GT3 (online supplementary table S1).5–7 40 However, while we found higher SVR12 rates in the overall and non-cirrhotic groups, and similar SVR12 rates in patients with cirrhosis treated by SOF+RBV and SOF+DCV regimens, we found lower SVR12 rates in patients with cirrhosis treated by SOF+RBV+Peg-IFN or SOF+DCV+RBV than results previously reported in clinical trials for GT3 patients.5 6 41 42 Since real-world studies are more likely to include patients with cirrhosis with more advanced liver disease than clinical trials, SVR12 rates among patients with cirrhosis in this real-world analysis may be lower than those seen in clinical trials as a result.
Interestingly, Peg-IFN was still used in more than one-third of the patients included in our meta-analysis. However, as the newer DAAs are being introduced to Asia, the use of Peg-IFN will be replaced by the much safer IFN-free DAA regimens that can produce the same or better efficacy. This is especially evident as current studies have demonstrated that newer DAA regimens such as SOF+VEL can lead to higher SVR12 for patients with cirrhosis than what was observed in this current real-world analysis where most of the patients with cirrhosis were treated with SOF+RBV±Peg-IFN.43 44 However, there are currently no data on the real-world SVR12 of SOF+VEL for GT3 in Asia, so further study is necessary.
In addition to SVR12, another important consideration is the impact of treatment on patient-reported outcomes (PROs). Studies on the impact of treatment with new DAAs on PROs found that the use of all oral IFN-free regimens was superior to those containing IFN.45–48 Given the similar SVR12 rates among patients treated with SOF+RBV and those with Peg-IFN-containing regimen, IFN-free therapies may be preferable with regard to patient tolerability and PROs.
There are several limitations to our study. Despite a comprehensive search, there were overall relatively few studies from Asia, with most studies coming from South Asia, such as India and Pakistan, and only two study from South-East Asia (Myanmar) and one study from West Asia (Iran), and no studies from high-income Asian countries such as Japan, Korea and Taiwan, which can be due to the relatively low frequency of GT3 in some of these Asian countries. Moreover, SOF+RBV±Peg-IFN was the most common therapy for patients with HCV GT3 in Asia, which accounted for 96% of all therapies in our analyses. Therefore, most of the comparisons were conducted between 24 weeks of SOF+RBV and 12 weeks of SOF+RBV+Peg-IFN. Also, since 5 of the 15 eligible studies’ data were published only as conference abstracts, limited descriptive data were available. In addition, only two studies reported AEs, and data on RBV or Peg-IFN dose modification or discontinuation and long-term outcomes were not available.
Heterogeneities were also high in the overall pooled SVR12 rate, but we performed several subgroup analyses and found that cirrhosis status contributed to approximately 69.5% of the observed heterogeneity. Prior treatment experience also contributed to heterogeneity, but in the analyses involving treatment-naïve cirrhotic and treatment-naive non-cirrhotic groups, heterogeneity was low (I2 was 17.2% and 10.4%). Finally, the number of patients with cirrhosis treated with SOF+RBV-based therapy was relatively small, with only 243 treatment-naïve and 87 treatment-experienced patients, with very limited data on compensated versus decompensated patients, both of which likely introduced bias leading to the unexpected observation of higher SVR12 in the treatment-experienced cirrhotic versus treatment-naïve cirrhotic groups. However, to overcome these potential biases we did conduct a sensitivity analysis which did not change our results, suggesting that the potential biases had little effect, but further studies with larger more diverse samples are needed in the future.
In conclusion, DAA therapy was very effective in treating patients with CHC GT3, with an overall SVR12 of 92.7%. For most patients, SOF+RBV therapy for 24 weeks was as effective as other DAA combinations, including 12 weeks of SOF+RBV+Peg-IFN, with significantly less AEs than IFN-contained therapies. However, SOF+RBV may not be optimal for patients with cirrhosis, with an SVR of 88.6% in our analysis, and newer DAAs should be considered for this population.
Footnotes
BW and FJ contributed equally.
Contributors: BW and FJ: study design, data collection, data analysis, data interpretation and drafting of the manuscript. YHY, EO, CDS, RCC: study design, data collection, data interpretation and critical revision of the manuscript. SD, ZL, NF: data collection, data interpretation and critical review of the paper. MHN: study conception, study design, data collection, data analysis, data interpretation, drafting of the manuscript and supervision of the study. All authors read and approved the final version of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: NF: grant/research support: MSD, Gilead Sciences, Bristol-Myers Squibb and Janssen Pharmaceuticals; speaker’s bureau: Gilead Sciences, MSD, Bristol Myers and Janssen Pharmaceuticals; advisory board: Gilead Sciences, AbbVie and Bristol-Myers Squibb. MHN: grant/research support: BK Kee Foundation, Asian Health Foundation, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, National Cancer Institute, Pfizer Pharmaceutical; advisory board member or consultant: Dynavax Laboratories, Gilead Sciences, Intercept Pharmaceuticals, Alnylam Pharmaceutical, Bristol-Myers Squibb, Novartis Pharmaceutical and Janssen Pharmaceuticals.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Presented at: This study has been presented as poster at the 2017 AASLD and 2018 APASL conferences.
References
- 1.Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–76. 10.1016/S2468-1253(16)30181-9 [DOI] [PubMed] [Google Scholar]
- 2.Petruzziello A, Marigliano S, Loquercio G, et al. . Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol 2016;22:7824–40. 10.3748/wjg.v22.i34.7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkontchou G, Ziol M, Aout M, et al. . HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat 2011;18:e516–e522. 10.1111/j.1365-2893.2011.01441.x [DOI] [PubMed] [Google Scholar]
- 4.Lawitz E, Mangia A, Wyles D, et al. . Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–87. 10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 5.Zeuzem S, Dusheiko GM, Salupere R, et al. . Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993–2001. 10.1056/NEJMoa1316145 [DOI] [PubMed] [Google Scholar]
- 6.Foster GR, Pianko S, Brown A, et al. . Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 2015;149:1462–70. 10.1053/j.gastro.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 7.Nelson DR, Cooper JN, Lalezari JP, et al. . All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015;61:1127–35. 10.1002/hep.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu ML, Chuang WL. New treatments for HCV: perspective from Asia. Clin Liver Dis 2015;5:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood A, Midha V, Mahajan R, et al. . Results of sofosbuvir-based combination therapy for chronic hepatitis C cohort of Indian patients in real-life clinical practice. J Gastroenterol Hepatol 2017;32:894–900. 10.1111/jgh.13628 [DOI] [PubMed] [Google Scholar]
- 10.Puri P, Saraswat VA, Dhiman RK, et al. . Indian National Association for Study of the Liver (INASL) Guidance for Antiviral Therapy Against HCV Infection: Update 2016. J Clin Exp Hepatol 2016;6:119–45. 10.1016/j.jceh.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umar M, Khaar HT, Akhter TS, et al. . Diagnosis, Management And Prevention Of Hepatitis C In Pakistan 2017. J Ayub Med Coll Abbottabad 2016;28(Suppl 1):S839–82. [PubMed] [Google Scholar]
- 12.Capileno YA, Van den Bergh R, Donchunk D, et al. . Management of chronic Hepatitis C at a primary health clinic in the high-burden context of Karachi, Pakistan. PLoS One 2017;12:e0175562 10.1371/journal.pone.0175562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omata M, Kanda T, Wei L, et al. . APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int 2016;10:702–26. 10.1007/s12072-016-9717-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asia. In: MeSH Unique ID: D001208: MeSH. [Google Scholar]
- 15.Sherman RE, Anderson SA, Dal Pan GJ, et al. . Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med 2016;375:2293–7. 10.1056/NEJMsb1609216 [DOI] [PubMed] [Google Scholar]
- 16.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael Borenstein LVH, Higgins JPT, Rothstein HR. Chapter 20: Meta-Regression Introduction to Meta-Analysis: John Wiley & Sons, Ltd, 2009:202. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ (Clinical research ed. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD. Meta-analysis: potentials and promise. BMJ 1997;315:1371–4. 10.1136/bmj.315.7119.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. 3.4.1 edn: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 22.Schwarzer G. meta: An R package for meta-analysis. In: R news 2007. [Google Scholar]
- 23.Hadley W. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag, 2009. [Google Scholar]
- 24.Wells GA SB, O’Connell D, Peterson J, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 25.Stovold E, Beecher D, Foxlee R, et al. . Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev 2014;3:54 10.1186/2046-4053-3-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbas Z, Saad M, Nadeem R, et al. . Sofosbuvir and ribavirin without or with pegylated interferon for hepatitis C genotype 3: A real world experience. Hepatol Int 2017;11:S1091. [Google Scholar]
- 27.Akhter TS, Umar M, Khaar HT, et al. . Sofosbuvir for the treatment of hepatitis C genotype 3 infected patients In Pakistan. J Ayub Med Coll Abbottabad 2016;28:S884–9. [PubMed] [Google Scholar]
- 28.Farooqi JI, Humayun M, Chaudhry A, et al. . Multi-center experience using Sofosbuvir & ribavirin with and without pegylated interferon to treat hepatitis C patients with and without liver cirrhosis (RESiP study: Real-life experience with sofosbuvir in Pakistan). Hepatology 2016;63:962A. [Google Scholar]
- 29.Goel A, Bhargava R, Rai P, et al. . Treatment of chronic genotype-3 hepatitis C virus infection using direct-acting antiviral agents: An Indian experience. Indian J Gastroenterol 2017;36:227–34. 10.1007/s12664-017-0763-3 [DOI] [PubMed] [Google Scholar]
- 30.Hlaing NKT, Mitrani RA, Aung ST, et al. . Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotypes 1-4 and 6 in Myanmar: Real-world experience. J Viral Hepat 2017;24:927–35. 10.1111/jvh.12721 [DOI] [PubMed] [Google Scholar]
- 31.Mehta R, Kabrawala M, Nandwani S, et al. . Efficacy and safety of sofosbuvir-based therapy for chronic hepatitis C infection in "real-life" cohort. Indian J Gastroenterol 2016;35:459–64. 10.1007/s12664-016-0713-5 [DOI] [PubMed] [Google Scholar]
- 32.Merat S, Sharifi AH, Haj-Sheykholeslami A, et al. . The efficacy of 12weeks of sofosbuvir, daclatasvir, and ribavirin in treating hepatitis C patients with cirrhosis, genotypes 1 and 3. Hepat mon 2017;17:1–4. [Google Scholar]
- 33.Sarwar S, Khan AA. Sofosbuvir based therapy in hepatitis C patients with and without cirrhosis: Is there difference? Pak J Med Sci 2017;33:37–41. doi:10.12669/pjms.331.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satsangi S, Mehta M, Duseja A, et al. . Dual treatment with sofosbuvir plus ribavirin is as effective as triple therapy with pegylated interferon plus sofosbuvir plus ribavirin in predominant genotype 3 patients with chronic hepatitis C. J Gastroenterol Hepatol 2017;32:859–63. 10.1111/jgh.13595 [DOI] [PubMed] [Google Scholar]
- 35.Shah S, Acharya SK, Mehta R, et al. . Sofosbuvir plus ribavirin in the treatment of chronic HCV infection in India. Hepatol Int 2016;10:S15. [Google Scholar]
- 36.Sidhu SS, Malhi NS, Goyal O, et al. . Treatment of chronic hepatitis C genotype 3 with Sofosbuvir-based therpy: a real-life study. Hepatol Int 2017;11:277–85. 10.1007/s12072-017-9794-1 [DOI] [PubMed] [Google Scholar]
- 37.Win KM, Hlaing NKT, Bwa AH, et al. . Treatment chronic hepatitis C Myanmar patients with sofosbuvir, pegylated interferon and ribavirin. Hepatol Int 2016;10:S132. [Google Scholar]
- 38.Yousuf MH, Yousaf MI, Iqbal S. Dramatic response of hepatitis C patients chronically infected with HCV genotype 3 to sofosbuvir-based therapies in Punjab, Pakistan. Hepatol int 2017;11:S27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goossens N, Negro F. Is genotype 3 of the hepatitis C virus the new villain? Hepatology 2014;59:2403–12. 10.1002/hep.26905 [DOI] [PubMed] [Google Scholar]
- 40.Berden FA, Aaldering BR, Groenewoud H, et al. . Identification of the best direct-acting antiviral regimen for patients with hepatitis C virus genotype 3 infection: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2017;15:349–59. 10.1016/j.cgh.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 41.Jacobson IM, Gordon SC, Kowdley KV, et al. . Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867–77. 10.1056/NEJMoa1214854 [DOI] [PubMed] [Google Scholar]
- 42.Lawitz E, Lalezari JP, Hassanein T, et al. . Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 2013;13:401–8. 10.1016/S1473-3099(13)70033-1 [DOI] [PubMed] [Google Scholar]
- 43.Sofosbuvir/Velpatasvir (Epclusa) for Hepatitis C. JAMA 2017;317:639–40. 10.1001/jama.2016.12279 [DOI] [PubMed] [Google Scholar]
- 44.von Felden J, Vermehren J, Ingiliz P, et al. . High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther 2018;47:1288–95. 10.1111/apt.14592 [DOI] [PubMed] [Google Scholar]
- 45.Umar M, Akhter TS, Akbar I, et al. . Role of generics in treatment of hepatitis c infection. J Ayub Med Coll Abbottabad 2016;28(Suppl 1):S890–4. [PubMed] [Google Scholar]
- 46.Younossi ZM, Stepanova M, Chan HL, et al. . Patient-reported outcomes in Asian patients with chronic hepatitis C treated with ledipasvir and sofosbuvir. Medicine 2016;95:e2702 10.1097/MD.0000000000002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 2014;46(Suppl 5):S186–S196. 10.1016/j.dld.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 48.Younossi ZM, Stepanova M, Nader F, et al. . The patient’s journey with chronic hepatitis C from interferon plus ribavirin to interferon- and ribavirin-free regimens: a study of health-related quality of life. Aliment Pharmacol Ther 2015;42:286–95. 10.1111/apt.13269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2018-000209supp001.pdf (322.4KB, pdf)