Abstract
Background
Non-US-born individuals account for the majority of active tuberculosis (TB) in the United States. Interferon gamma release assay (IGRA) is the preferred diagnostic test for latent TB but can produce an indeterminate result. We investigated the prevalence and predictors of an indeterminate IGRA (IND-IGRA) in a diverse cohort of non-US-born individuals and evaluated outcomes after IND-IGRA.
Methods
We identified patient age ≥18 years who had an outpatient IGRA between 2010 and 2017 in our health system and whose primary language was not English. We used univariate and multivariable logistic regression to examine the association of IND-IGRA with a variety of clinical factors.
Results
Of 3128 outpatients with ≥1 IGRA done, 33% were Asian, 30% Hispanic, and 29% black; 44% were men, and the median age was 50 years. An initial IND-IGRA occurred in 118 (3.8%; 95% confidence interval [CI], 3.1%–4.5%); notably, Asian race (55%) and rheumatologic conditions (25%) were prevalent in this group. In multivariable analysis, Asian race was independently associated with IND-IGRA (adjusted odds ratio [aOR], 2.9; 95% CI, 1.9–4.3), in addition to the presence of anemia and hypoalbuminemia (aOR for interaction, 4.3; 95% CI, 1.3–14.3). Only 55% of patients with an initial IND-IGRA underwent repeat testing; of those who did, 66% had a determinate result.
Conclusions
Asian race and anemia/hypoalbuminemia were independent risk factors for an indeterminate IGRA outcome in foreign-born patients screened in the United States. Our study underscores the importance of following through on indeterminate results in these key subgroups.
Keywords: foreign-born, interferon gamma release assay, indeterminate, latent tuberculosis
Early detection and treatment of latent tuberculosis infection (LTBI) can reduce the risk of progression to tuberculosis (TB) disease, which remains a significant source of morbidity and mortality worldwide. The majority of active TB in the United States occurs among non-US-born individuals [1], and the incidence of active TB in persons from high-burden countries appears to be highest during the first 2 years after immigration [2]. The interferon gamma release assay (IGRA) is the preferred diagnostic tool for LTBI in most settings because of its greater specificity compared with TB skin testing (TST) and the provision of a result without another clinical visit. IGRA is recommended over TST for persons who have received the Bacille Calmette-Guérin (BCG) vaccine or who are less likely to return to have the TST read [3]. Mexico and nearly all countries in Asia, Africa, South America, and Eastern Europe have current national policies recommending BCG vaccination [4].
Two IGRA tests, Quantiferon-TB Gold InTube (Cellestis Limited) and T-SPOT.TB (Oxford Immunotec Limited), are Food and Drug Administration–approved and recommended for LTBI testing [3]. The IGRA blood test measures the T-cell immune response to Mycobacterium tuberculosis–specific antigens and requires an adequate host immune response to a mitogen (positive control) and a sufficiently low level of background response (negative control/nil) to yield a valid result. Though widely used in the United States over the past decade, IGRA has not been studied extensively in large cohorts of US-based, non-US-born populations, including those with diverse chronic conditions, who have a high prevalence of LTBI and are more likely to benefit from testing and treatment [5]. Most of the existing literature on IGRA in the foreign-born US population comes from public health clinics with limited information on concurrent medical conditions or with a focus on subpopulations such as immigrants and refugees suspected to have active or latent TB [6, 7].
A drawback of IGRA testing is that the test result may be indeterminate. An indeterminate outcome may result from an insufficient immune response to the mitogen control (low mitogen) or too high a level of background immune activity (high nil). Previous studies have found rates of indeterminate IGRA between 2% and 11%, with higher rates in populations with chronic conditions such as rheumatologic or immunosuppressive states, and often with low mitogen as the underlying reason for the indeterminate result [6–10]. Uncertainty exists as to the next steps following an indeterminate IGRA result, particularly in individuals with a moderately high pretest probability of LTBI, such as persons born in TB-endemic countries. Identification of potential risk factors for an indeterminate IGRA result in non-US-born persons and description of clinical outcomes following an indeterminate result can inform the optimal strategy for latent TB diagnosis in this key population.
We conducted a retrospective cohort study to estimate the prevalence and risk factors of indeterminate IGRA in a diverse outpatient population of non-US-born patients from countries with high TB incidence and to describe the outcomes of those individuals with indeterminate IGRA results. We assessed whether TB testing was repeated and/or whether TB treatment initiated and evaluated progression to active TB in these individuals.
METHODS
Study Population
Quantiferon Gold-InTube (QFT-GIT) was first introduced in 2009 for routine use in the University of Washington (UW) Health System, comprising 2 large academic medical centers (Harborview Medical Center and University of Washington Medical Center) and an extended network of primary care and specialty clinics in King County, Washington. In 2015, King County’s non-US-born population was estimated to be 21%, compared with 13% nationally, which makes the UW Health System particularly relevant for this analysis [11, 12]. IGRA was rapidly adopted within the UW Health System for LTBI testing after its introduction, though providers could elect to use TST per individual or patient preference. IGRA testing was recommended in accordance with the American Thoracic Society guidelines. We used Leaf to access a clinical data repository of electronic health records to identify foreign-born persons eligible for screening with IGRA. Leaf is a self-service, web-based data tool developed to facilitate clinical research and quality improvement projects within the UW Health System, which draws raw data from fixed data fields in the electronic medical records (EMRs). Laboratory results and their interpretations were available from 2010 to the present, and demographic data as recorded in the EMR were also available. Primary language was routinely collected from patients registering in the UW Health System.
We included all patients 18 years of age and older who had an IGRA collected in an outpatient setting between January 2010 and July 2017 and had a non-English primary language, as a surrogate for non-US-born status, which has been validated and shown to be a specific marker [13]. We excluded individuals whose primary languages represented countries with low TB incidence (<10 per 100 000 person-years), determined by World Health Organization TB Country Profiles [14]. For patients with >1 IGRA, we analyzed the first outpatient IGRA performed. We selected specific risk factors for indeterminate IGRA outcome based on their significance in prior studies and their availability and reliability in our clinical database. For example, anemia and hypoalbuminemia have been shown to be associated with indeterminate IGRA results in studies of Sub-Saharan African refugees and of patients with inflammatory bowel disease, respectively, and our study aimed to elucidate whether such associations were reproducible in our general, demographically and clinically heterogeneous, non-US-born population [15–17].
The study was approved by the University of Washington Institutional Review Board.
Data Sources and Definitions
Patient demographic data, laboratory values, and test dates were extracted. The outcome of interest, indeterminate IGRA (IND-IGRA), was determined by laboratory interpretation and confirmed by our own calculation based on 3 raw tube values (for response to TB antigen, mitogen, and nil), derived from the algorithm published in the Centers for Disease Control and Prevention (2010) guidelines for interpretation of IGRA and QuantiFERON-TB Gold QFT ELISA Package Insert [18, 19]. We examined the following covariates for association with the outcome IND-IGRA: age, sex, race, diabetes mellitus, chronic kidney disease, anemia, hypoalbuminemia, liver disease and HIV infection, and CD4 count <200 cells/mm3 if HIV-infected. We examined age at the time of IGRA testing as a continuous and categorical variable (18–40, 40–65, and >65 years). We categorized race/ethnicity as listed in the database or extrapolated from unambiguous language and country of origin data as follows: Asian/Pacific Islander (PI; hereinafter referred to as simply Asian), black, Hispanic (Spanish primary language), and other (which included Caucasian and Native American individuals). We chose Hispanic as a referent group because Caucasians were underrepresented in our cohort.
Diabetes was defined as presence of ≥1 hemoglobin A1C >6.5%. Renal disease was determined by creatinine clearance (estimated glomerular filtration rate) using the CKD-EPI equation [20] and was analyzed at <60 mL/min and <30 mL/min. We defined anemia as a hemoglobin (Hb) <13 g/dL for men and <12 g/dL for women. Hypoalbuminemia was defined as <3.5 g/dL. Liver disease was defined as AST–platelet ratio index (APRI) >1 [21, 22]. HIV status was determined as positive for any positive antigen–antibody test present before or up to 30 days after the IGRA or any positive Western blot before or up to 1 year after the IGRA. A patient was also considered HIV-positive by any positive HIV RNA value before or within 1 year after IGRA testing or if any RNA testing (negative or positive) occurred more than once and fell in the above window.
Laboratory time frames with respect to IGRA date were selected to reflect subjects’ most probable clinical status at the time of the IGRA. Patients with any hemoglobin A1C >6.5% over the 7-year span of the study were considered diabetic, whereas those with negative or missing A1C were considered nondiabetic. For other labs, we selected laboratory results closest to the date of the IGRA, within 1 year before, or up to 6 months after; results outside of this time frame were assigned as missing. We also examined the reason for IND-IGRA: either failure to generate response to mitogen control (low positive control response) or too high a level of background (high negative control response).
Our primary focus was on outpatient IGRA results because IGRA was developed as an outpatient screening test [4] and acute illness requiring hospitalization may be a risk factor for indeterminate results [23]. We further characterized the outpatient IND-IGRA cohort through chart review to assess concurrent medical conditions, to determine whether any subsequent TB testing by IGRA or PPD was conducted following an indeterminate result, to determine whether treatment for LTBI was initiated, and to document TB-free survival. We also assessed concomitant corticosteroid and select disease-modifying agent use in persons with IND-IGRA results and known rheumatologic conditions. Additional details on variable selection and definitions are available in the Supplementary Materials. Data identified through chart review were entered into a standardized data collection form using Research Electronic Data Capture (REDCap) [24].
Statistical Analyses
We calculated the prevalence and confidence intervals (CIs) of IND-IGRA in our main outpatient cohort and in inpatients who had been excluded from our main cohort. We used χ2 testing to examine univariate associations and multivariable logistic regression to determine factors associated with IND-IGRA. We included factors of a priori interest (age, sex) based on the currently available literature [6, 25] and those associated with IND-IGRA in univariate analysis (at a significance level of P < .05) in the multivariable model. We also created an indicator variable for race (Asian [vs non-Asian]) for inclusion in the final model. We explored the possibility of effect modification between anemia and hypoalbuminemia by including an interaction term in the model. To examine whether missing data may have introduced bias, we conducted a sensitivity analysis using multiple imputation to account for missing laboratory data. We imputed 20 data sets with substantive model-compatible fully conditional specification [26], imputing the following partially observed variables: anemia, hypoalbuminemia, anemia–hypoalbuminemia (indicator for both), and liver disease. All analyses were performed using Stata, IC 15 (College Station, TX).
RESULTS
Study Patients
Between 2010 and 2017, a total of 3128 patients who met age and foreign-born language criteria had ≥1 outpatient IGRA performed (Table 1). Our cohort was 33% Asian, 30% Hispanic, and 29% black; 44% were men, and the median age was 50 years. Seventy-three languages were represented, the most common being Spanish (30%), Amharic (11%), Vietnamese (9.2%), Somali (7.8%), and Mandarin (4.4%). A total of 2062 patients (66%; 95% CI, 64%–68%) had a negative IGRA, and 948 (30%; 95% CI, 27%–32%) had a positive IGRA.
Table 1.
Baseline Characteristics of Patients With and Without an Indeterminate IGRA
| Characteristics, No. (%) | Indeterminate IGRA (n = 118) |
Determinatea IGRA (n = 3010) |
P Value |
|---|---|---|---|
| Age, years | |||
| 18–39 | 30 (25) | 893 (30) | |
| 40–65 | 61 (52) | 1528 (51) | |
| >65 | 27 (23) | 589 (20) | .28 |
| Female | 72 (61) | 1673 (56) | .24 |
| Race/ethnicity | |||
| Asian/PI | 65 (55) | 970 (32) | |
| Black | 25 (21) | 881 (29) | |
| Hispanic | 23 (19) | 910 (30) | |
| Other | 5 (4) | 227 (8) | <.001 |
| Anemiab | 66/112 (59) | 751/2599 (29) | <.001 |
| Hypoalbuminemiac | 43/108 (40) | 276/2479 (11) | <.001 |
| Anemia + hypoalbuminemia | 38/107 (36) | 167/2355 (7) | <.001 |
| Liver diseased | 21/106 (20) | 124/2338 (5.3) | <.001 |
| Diabetes mellituse | 19/118 (16) | 474/3010 (16) | .92 |
| Chronic kidney disease | |||
| GFR < 60 | 18/111 (16) | 353/2670 (13) | .36 |
| GFR < 30 | 11/111 (10) | 222/2670 (8.3) | .55 |
| HIV infection | 15/118 (13) | 304/3010 (10) | .36 |
Abbreviations: GFR, glomerular filtration rate; IGRA, interferon gamma release assay; PI, Pacific Islander.
aPositive or negative.
bHemoglobin <13 g/dL if male and <12 g/dL if female.
cSerum albumin <3.5 g/dL.
dAST–platelet ratio index >1.
eHemoglobin A1C >6.5%.
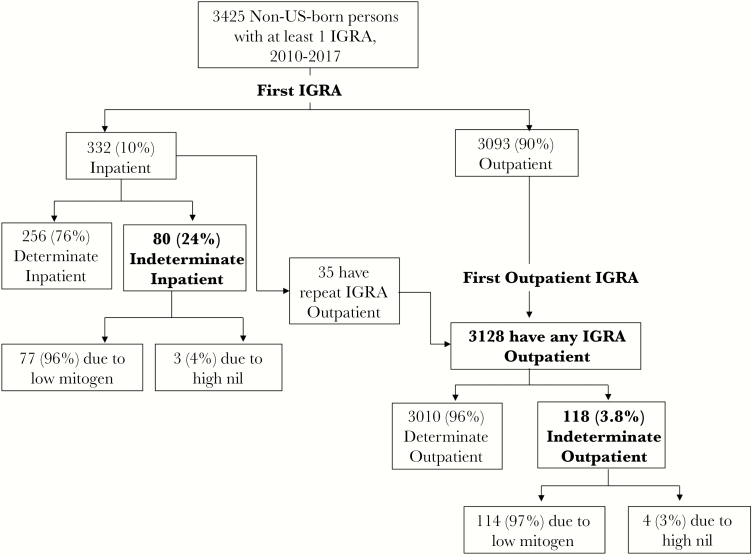
A total of 118 patients had an initial IND-IGRA, for a prevalence of 3.8% (95% CI, 3.1%–4.5%). Of the IND-IGRAs, nearly all (114, 97%) were due to low mitogen, and 4 (2.6%) were due to high nil (Figure 1). Patients with IND-IGRA were more likely than those with determinate IGRA to be Asian (55% vs 32%; P < .001). They were also more likely to be anemic, hypoalbuminemic, or both and to have an elevated APRI >1, which is suggestive of liver disease (Table 1).
Figure 1.
Interferon gamma release assay (IGRA) results among non-US-born persons in outpatient and inpatient settings.
IND-IGRA in Inpatients
Ten percent of all IGRA tests in foreign-born adults were performed in the inpatient setting (Figure 1). Inpatients had a significantly higher rate of IND-IGRA compared with outpatients. The prevalence of IND-IGRA was 24% (80/332; 95% CI, 20%–29%) among inpatients compared with 3.8% among outpatients (odds ratio [OR], 8.2; 95% CI, 6.0–11).
Risk Factors for IND-IGRA in Outpatients
In univariate analysis, variables associated with IND-IGRA were Asian race (OR, 2.7; 95% CI, 1.6–4.3), anemia (OR, 3.5; 95% CI, 2.4–5.2), hypoalbuminemia (OR 5.3; 95% CI, 3.5–7.9), and liver disease (OR, 4.4; 95% CI, 2.6–7.3). Age, sex, and other race/ethnicity, as well as diabetes, chronic kidney disease, and HIV infection, were not associated with IND-IGRA. Among people with HIV infection, there was no statistically significant difference in the proportion with a CD4 count <200 cells/mm3 between those with an indeterminate vs determinate IGRA. However, CD4 data within 1 year before or 6 months after IGRA testing were only available for 47% of HIV-infected individuals.
In the primary multivariable analysis, we evaluated all outpatients for whom complete laboratory and demographic data were available (Table 2). Asian race (referent: Hispanic; adjusted odds ratio [aOR], 2.9; 95% CI, 1.9–4.3) was independently associated with IND-IGRA. Asians accounted for 55% of IND-IGRAs, and their rate of IND-IGRA was 5.3% (95% CI, 4.0%–6.9%). Anemia, hypoalbuminemia, and liver disease, though each was associated with IND-IGRA in univariate analysis, were not associated when adjusted for other factors. There was, however, significant effect modification between anemia and hypoalbuminemia (aOR for interaction, 4.3; 95% CI, 1.3–14.3). Those with anemia and hypoalbuminemia accounted for 32% of all patients with IND-IGRAs, and their rate of IND-IGRA was 19% (95% CI, 13%–25%).
Table 2.
Factors Associated With an Indeterminate IGRA
| Factor | Crude OR (95% CI) | P Value | Adjusted ORa (95% CI) | P Value |
|---|---|---|---|---|
| Male sex | 0.80 (0.55–1.2) | .24 | 0.72 (0.48–1.1) | .12 |
| Age, by deciles | 1.07 (0.96–1.2) | .22 | 0.91 (0.80–1.0) | .19 |
| Race/ethnicity (ref: Hispanic) | ||||
| Black | 1.1 (0.63–2.0) | .69 | – | – |
| Asian/Pacific Islander | 2.7 (1.6–4.3) | <.001 | 2.9 (1.9–4.3) | <.001 |
| Other | 0.87 (0.33–2.3) | .78 | – | – |
| Anemiab | 3.5 (2.4–5.2) | <.001 | 1.5 (0.9–2.6) | .11 |
| Hypoalbuminemiac | 5.3 (3.5–7.9) | <.001 | 1.3 (0.47–3.9) | .54 |
| Anemia × hypoalbuminemia | 3.4 (1.05–11) | .041 | 4.3 (1.3–14.3) | .03 |
| Liver diseased | 4.4 (2.6–7.3) | <.001 | 1.5 (0.8–2.7) | .21 |
Abbreviations: CI, confidence interval; IGRA, interferon gamma release assay; OR, odds ratio.
aMultivariable model included all the covariates noted above (note: race/ethnicity examined as an indicator variable [Asian] in this model).
bHemoglobin <13 g/dL if male and <12 g/dL if female.
cSerum albumin <3.5 g/dL.
dAST–platelet ratio index >1.
We conducted a second multivariable analysis, using multiple imputation to account for missing data that included all 3128 individuals who underwent outpatient IGRA testing. This model identified anemia as an independent predictor of IND-IGRA (aOR, 1.7; 95% CI, 1.04–2.8), in addition to the combination of anemia and hypoalbuminemia, and Asian race (Supplementary Table A). The risk estimates of other covariates from the model with imputed data were similar to those of the primary model.
Follow-up After an Indeterminate Result
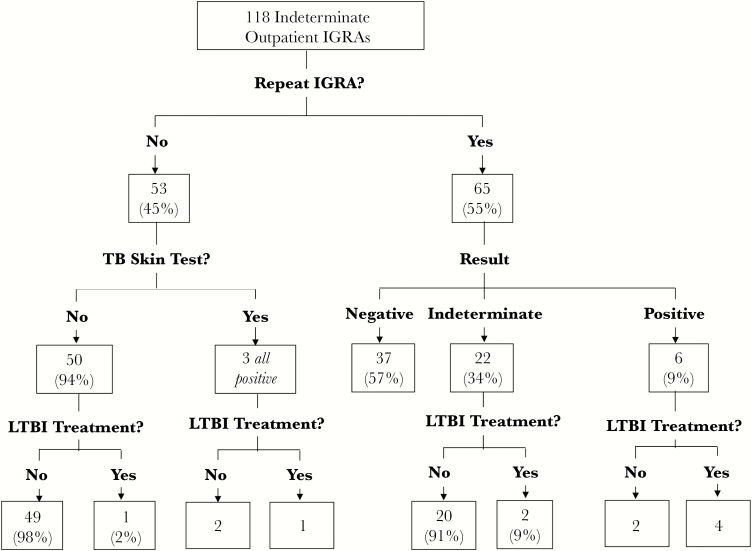
Among persons who had an IND-IGRA result, the median follow-up time between the first outpatient IGRA and the most recent clinic encounter or death (interquartile range [IQR]) was 2.1 (0.78–3.9) years. A total of 53 individuals (45%) did not receive follow-up IGRA testing (Figure 2). Of these, 3 people were tested with tuberculin skin test, all of whom tested positive. One declined treatment, and 2 were treated for LTBI with isoniazid. Another person who received no follow-up testing of any modality was instead treated preemptively for LTBI with isoniazid.
Figure 2.
Testing outcomes after an initial indeterminate interferon gamma release assay (IGRA) result.
The remaining 65 (55%) of IND-IGRA patients were retested by IGRA in an outpatient setting. On repeat testing, 37 (57%) had negative IGRA, 6 (9%) were positive, and 22 (34%) were again indeterminate. Treatment (isoniazid or isoniazid and rifapentine) was initiated in 4 out of the 6 repeat-positive IGRA cases and in 2 out of the 22 repeat-indeterminate cases.
Of the 2 repeat-IGRA-positive patients who were not treated, 1 refused treatment, and the other reported he was already treated for LTBI in his country of origin. The median time between initial and follow-up IGRAs (IQR) was 70 (23–245) days.
One patient in the IND-IGRA cohort developed active, culture-confirmed pulmonary TB, which occurred 6 months after an indeterminate IGRA that was not followed by retesting or treatment. This patient had HIV infection and a CD4 count of 34 cells/mm3 2 weeks before the IGRA testing and 36 cells/mm3 1 month before active TB was diagnosed.
Among the IND-IGRA patients, a total of 30 (25%) had a concurrent rheumatologic condition, 18 (15%) had end-stage liver disease (ESLD), and 8 (6.8%) had cancer. A total of 38 (32%) had a chronic condition that did not fall into 1 of these categories, including end-stage renal disease, diabetes mellitus, or HIV infection. Of the patients with rheumatologic conditions, a total of 18 (60%) were on systemic glucocorticoids; of those not on corticosteroids, 4 were on disease-modifying antirheumatic drugs (methotrexate, mycophenolate mofetil, or azathioprine).
DISCUSSION
Among foreign-born patients undergoing outpatient LTBI testing, we observed a 3.8% rate of indeterminate IGRA results, approximately twice the reported rate in studies examining IGRA performance in large US immigrant populations at public health clinics [6, 7]. Our higher rate may have been due to the fact that non-US-born persons screened in our health system were more likely to have chronic conditions associated with IND-IGRA, as our cohort is enriched with a specialty clinic population that is also more likely to be tested for LTBI.
Among our outpatient cohort, we found that Asian race was an independent risk factor for IND-IGRA and accounted for the majority of foreign-born outpatients with indeterminate results. In 2 prior studies of immigrants screened in public health clinics, Asian race was also found to be a risk factor for IND-IGRA [6, 27]. Genetic polymorphisms in T-lymphocyte and natural killer cell response to the mitogen control, phytohemagglutinin (PHA), may account for this finding. Genetic loci associated with differences in lymphocyte responses to PHA have been described [28, 29]. Notably, Asians represent the largest subgroup of foreign-born persons living in the United States at risk of TB [30] and the group with the highest incidence of active TB cases in the United States each year from 2012 to 2015 [1], which underscores the importance of following through on indeterminate results in this subpopulation in particular.
The combination of anemia and hypoalbuminemia was also an independent risk factor for IND-IGRA in our study. This combination may occur in diseases of chronic inflammation, including inflammatory bowel disease or systemic lupus erythematous. It may also occur in end-stage renal or liver disease and certain cancers. Patients with such conditions form a key population for LTBI testing and treatment due to increased use of immunosuppressant therapies, which increase the risk of reactivation TB. Anemia and hypoalbuminemia in combination may represent an overall inflammatory state contributing to an impaired immune response to the positive mitogen control. The Th17 inflammatory pathway, which has a key role in the pathogenesis of rheumatoid arthritis (RA), also downregulates the Th1 pathway responsible for generating interferon gamma in response to pathogens such as fungi and mycobacteria [31, 32]. Neutrophils also play a key role in RA and inflammatory bowel disease; an increased neutrophil-to-lymphocyte ratio has been shown to be associated with IND-IGRA due to insufficient mitogen response [33–35]. Individuals with chronic inflammatory conditions may also be on immunosuppressant therapy, such as systemic corticosteroids, which was observed in 60% of IND-IGRA patients in our cohort with rheumatologic conditions at the time of IGRA testing and which may also have influenced the IGRA result [8]. The combination of anemia and hypoalbuminemia may also represent functional immune compromise in individuals with these chronic conditions, by way of decreased absolute lymphocyte count [36].
Although other studies have found advanced age to be a risk factor for indeterminate IGRA, age was not a significant risk factor in our clinical cohort. This may be due to selection by indication bias, in which an important indication for IGRA screening was preparation to start immunosuppressive therapy, and underlying comorbidities were seen at all ages.
It was concerning that nearly half of the individuals who had an initial IND-IGRA did not receive follow-up IGRA screening or LTBI treatment. During the study time period, 1 IND-IGRA case with HIV infection did progress to active pulmonary TB, underscoring the risk inherent in inaction after an IND-IGRA result in patients with moderate or higher pretest probability for LTBI and underlying risk factors for active TB progression. There is currently no consensus on the optimal strategy following an IND-IGRA result. Other studies have shown that, on repeat testing, a substantial majority of IND-IGRAs are later found to be negative [7, 8]. In our study, of those with an initial IND-IGRA who received repeat testing, 66% had a determinate result the second time, and most of these (57%) were indeed negative. Laboratory error or specimen mishandling may cause an indeterminate result, which may resolve in a repeat test. However, in our system, such error, when detected, is noted by the laboratory in the interpretation field or absence of raw tube values, and these IGRAs were not included in our analyses. It is not possible to estimate the contribution of undetected laboratory error to the proportion of indeterminate results observed, but we expect that it is low. Due to repeated observations of indeterminate QFT-GIT results in persons evaluated for liver transplant, our institution’s solid organ transplant candidate evaluation program instituted an algorithm of reflexive testing by T-SPOT.TB IGRA in persons with an IND-IGRA by QFT-GIT. This test has the theoretical advantages of adjusting for lymphopenia and removing responding T cells from the whole-blood milieu associated with other pathologic states [4].
Although there is insufficient evidence to recommend a specific test or interval frequency of repeat testing if an IND-IGRA result occurs, the likelihood of an interpretable result on repeat testing suggests that repeat testing should be performed, especially in a population at moderate risk for latent TB infection. Increased provider education about an indeterminate test result may improve the rate of follow-up testing.
Finally, we found an increased rate of IND-IGRA among inpatients compared with outpatients, suggesting a lower utility of IGRA in hospitalized patients. IGRA is not intended as a diagnostic test for active TB, and our findings emphasize its limitations as a test for detection of TB infection in the setting of hospitalization.
Our study has strengths and limitations. Our cohort included a large, diverse group of patients representing nearly 100 countries of origin with a variety of clinical risk factors, which enabled us to evaluate multiple predictors of indeterminate results. This was the first study to our knowledge to evaluate outcomes of non-US-born US individuals following an indeterminate result. Limitations include the use of non-English language as an indicator for non-US-born status, which only captures a portion of all such individuals and likely selects for individuals who have more recently moved to the United States. Recent exposure to a high-TB environment has been shown to increase the risk for new active TB, which makes this population of special interest, but it also limits the generalizability of our findings [2]. Finally, our analysis represented a secondary use of a clinical database, which is inherently vulnerable to misclassification and incomplete data. However, we sought to mitigate the former by using objective contemporaneous laboratory values when feasible instead of diagnostic codes for disease conditions, which are subject to unpredictable misclassification and incompleteness. Although complete laboratory data were not available for all patients, the multiple imputation analysis suggested that missing data did not introduce significant bias.
In conclusion, Asian race and the combination of anemia/hypoalbuminemia were independent risk factors for an indeterminate IGRA outcome in our non-US-born population. Only 55% of those with an initial IND-IGRA received repeat TB testing, even though this group was at moderate risk for LTBI and reactivation. Additional research on predictors of and outcomes after IND-IGRA would help to establish guidelines for retesting and treatment.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Nicholas Dobbins and the Leaf support team at the University of Washington Institute of Translational Health Sciences (UW ITHS) for their help and the staff of the University of Washington Department of Laboratory Medicine for test performance. We thank Thomas Carr for his assistance in data management and Dr. Chetan Seshadri for his review of our work.
Financial support. The UW ITHS grant UL1TR000423 from the National Center for Research Resources/National Institutes of Health (NIH) and ITHS grant UL1 TR002319 supported this work, as well as National Institute of Allergy and Infectious Diseases/NIH T32 AI07140 to A.E.S.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Salinas JL, Mindra G, Haddad MB, et al. Leveling of tuberculosis incidence - United States, 2013-2015. MMWR Morb Mortal Wkly Rep 2016; 65:273–8. [DOI] [PubMed] [Google Scholar]
- 2. Cohen T, Murray M. Incident tuberculosis among recent US immigrants and exogenous reinfection. Emerg Infect Dis 2005; 11:725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med 2011; 8:e1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell JR, Chen W, Johnston J, et al. Latent tuberculosis infection screening in immigrants to low-incidence countries: a meta-analysis. Mol Diagn Ther 2015; 19:107–17. [DOI] [PubMed] [Google Scholar]
- 6. Banach DB, Harris TG. Indeterminate QuantiFERON®-TB Gold results in a public health clinic setting. Int J Tuberc Lung Dis 2011; 15:1623–30. [DOI] [PubMed] [Google Scholar]
- 7. Blount RJ, Tran MC, Everett CK, et al. Tuberculosis progression rates in U.S. immigrants following screening with interferon-gamma release assays. BMC Public Health 2016; 16:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsia EC, Schluger N, Cush JJ, et al. Interferon-γ release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum 2012; 64:2068–77. [DOI] [PubMed] [Google Scholar]
- 9. Kaur M, Singapura P, Kalakota N, et al. Factors that contribute to indeterminate results from the QuantiFERON-TB gold in-tube test in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017; pii: S1542–3565(17)31411–8. [DOI] [PubMed] [Google Scholar]
- 10. Richeldi L, Losi M, D’Amico R, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 2009; 136:198–204. [DOI] [PubMed] [Google Scholar]
- 11. King County, Washington State. Demographic trends of King County Available at: https://www.kingcounty.gov/independent/forecasting/KingCountyEconomicIndicators/Demographics.aspx. Accessed 18 January 2018.
- 12. Pew Research Center. Facts of U.S. immigrants, 2015 Available at: http://www.pewhispanic.org/2017/05/03/facts-on-u-s-immigrants-current-data/. Accessed 18 January 2018.
- 13. Levison J, Triant V, Losina E, et al. Development and validation of a computer-based algorithm to identify foreign-born patients with HIV infection from the electronic medical record. Appl Clin Inform 2014; 5:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Tuberculosis country profiles Available at: http://www.who.int/tb/country/data/profiles/en/. Accessed 21 May 2018.
- 15. Banfield S, Pascoe E, Thambiran A, et al. Factors associated with the performance of a blood-based interferon-γ release assay in diagnosing tuberculosis. PLoS One 2012; 7:e38556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papay P, Eser A, Winkler S, et al. Predictors of indeterminate IFN-γ release assay in screening for latent TB in inflammatory bowel diseases. Eur J Clin Invest 2011; 41:1071–6. [DOI] [PubMed] [Google Scholar]
- 17. Shu CC, Wu VC, Yang FJ, et al. Predictors and prevalence of latent tuberculosis infection in patients receiving long-term hemodialysis and peritoneal dialysis. PLoS One 2012; 7:e42592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazurek GH, Jereb J, Vernon A, et al. ; IGRA Expert Committee; Centers for Disease Control and Prevention Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010; 59:1–25. [PubMed] [Google Scholar]
- 19. Qiagen. Quantiferon-TB Gold package insert 2016. Available at: http://www.quantiferon.com/us/products/quantiferon-tb-gold/. Accessed 18 May 2018.
- 20. National Kidney Foundation. CKD-EPI creatinine equation 2009. Available at: https://www.kidney.org/content/ckd-epi-creatinine-equation-2009. Accessed 18 May 2018.
- 21. Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53:726–36. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz Y, Yonal O, Kurt R, et al. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): usefulness in patients with chronic liver disease: APRI in chronic liver disease. Hepat Mon 2011; 11:103–6. [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrara G, Losi M, Meacci M, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005; 172:631–5. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tebruegge M, de Graaf H, Sukhtankar P, et al. Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J Clin Microbiol 2014; 52:2694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartlett JW, Seaman SR, White IR, Carpenter JR; Alzheimer’s Disease Neuroimaging Initiative Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res 2015; 24:462–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson T, Tomaro J, Jobb C. Implementation of an interferon-gamma release assay to screen for tuberculosis in refugees and immigrants. J Immigr Minor Health 2013; 15:686–92. [DOI] [PubMed] [Google Scholar]
- 28. Carreras-Sureda A, Rubio-Moscardo F, Olvera A, et al. Lymphocyte activation dynamics is shaped by hereditary components at chromosome region 17q12-q21. PLoS One 2016; 11:e0166414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walldén J, Ilonen J, Roivainen M, et al. ; ABIS Study Group Effect of HLA genotype or CTLA-4 polymorphism on cytokine response in healthy children. Scand J Immunol 2008; 68:345–50. [DOI] [PubMed] [Google Scholar]
- 30. Lopez G, Ruiz NG, Patten E. Key facts about Asian Americans, a diverse and growing population 2017. Available at: http://www.pewresearch.org/fact-tank/2017/09/08/key-facts-about-asian-americans/. Accessed 11 January 2018.
- 31. Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum 2010; 62:2876–85. [DOI] [PubMed] [Google Scholar]
- 32. Zelante T, De Luca A, Bonifazi P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 2007; 37:2695–706. [DOI] [PubMed] [Google Scholar]
- 33. Wera O, Lancellotti P, Oury C. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 2016; 5(12):pii: E118. doi:10.3390/jcm5120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woo KS, Kim BG, Choi JL, et al. Neutrophil-to-lymphocyte ratio is associated with impaired interferon-gamma release to phytohemagglutinin. PLoS One 2015; 10:e0125794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10:593–601. [DOI] [PubMed] [Google Scholar]
- 36. Jung HJ, Kim TJ, Kim HS, et al. Analysis of predictors influencing indeterminate whole-blood interferon-gamma release assay results in patients with rheumatic diseases. Rheumatol Int 2014; 34:1711–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.