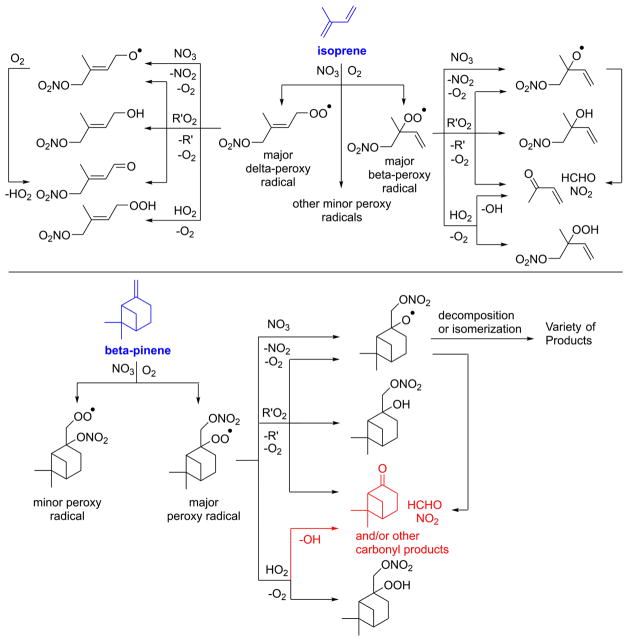
Figure 2.
Condensed reaction mechanism for isoprene and β-pinene oxidation via NO3 (adapted from Schwantes et al., 2015 and Boyd et al., 2015). For brevity, only products generated from the dominant peroxy radicals (RO2) are shown. R′ represents an alkoxy radical, carbonyl compound, or hydroxy compound. Two of the largest uncertainties in β-pinene oxidation are shown in red: (1) quantification of product yields from the RO2+ HO2 channel and (2) identification of carbonyl products formed from RO2 reaction with NO3, RO2, or HO2 (see text for more details).