Table 1.
Reaction rate constants of NO3+ BVOC.
| Compound | k(NO3+BVOC) (cm3 molecule−1 s−1)a | Temperature (K) | Technique/reference |
|---|---|---|---|
|
| |||
Isoprene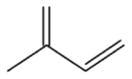
|
(5.94 ± 0.16)×10−13 | 295 | RR/(Atkinson et al., 1984) |
| (1.30 ± 0.14)×10−12 | 298 | DF-MS/(Benter and Schindler, 1988) | |
| (3.03 ± 0.45)×10−12exp[−(450 ± 70)/T] | 251–281 | F-LIF/(Dlugokencky and Howard, 1989) | |
| (6.52 ± 0.78)×10−13 | 297 | F-LIF/(Dlugokencky and Howard, 1989) | |
| (1.21 ± 0.20)×10−12 | 298 | RR/(Barnes et al., 1990) | |
| (7.30 ± 0.44)×10−13 | 298 | DF-MS/(Wille et al., 1991) | |
| (8.26 ± 0.60)×10−13 | 298 | DF-MS/(Wille et al., 1991) | |
| (1.07 ± 0.20)×10−12 | 295 | PR-A/(Ellermann et al., 1992) | |
| (6.86 ± 0.55)×10−13 | 298 | RR/(Berndt and Boge, 1997b) | |
| (7.3 ± 0.2)×10−13 | 298 | F-CIMS/(Suh et al., 2001) | |
| (6.24 ± 0.11)×10−13 | 295 | RR/(Zhao et al., 2011b) | |
| 6.5×10−13 (Δlog k: ± 0.15) | 298 | IUPAC | |
|
| |||
α-pinene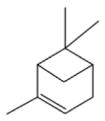
|
(5.82 ± 0.16)×10−12 | 295 | RR/(Atkinson et al., 1984) |
| (1.19 ± 0.31)×10−12exp[(490 ± 70)/T] | 261–383 | F-LIF/(Dlugokencky and Howard, 1989) | |
| (6.18 ± 0.94)×10−12 | 298 | F-LIF/(Dlugokencky and Howard, 1989) | |
| (6.56 ± 0.94)×10−12 | 298 | RR/(Barnes et al., 1990) | |
| (3.5 ± 1.4)×10−13exp[(841 ± 144)/T] | 298–423 | DF-LIF/(Martinez et al., 1998) | |
| (5.9 ± 0.8)×10−12 | 298 | DF-LIF/(Martinez et al., 1998) | |
| (5.82 ± 0.56)×10−12 | 298 | RR/(Kind et al., 1998) | |
| (4.88 ± 0.46)×10−12 | 298 | RR/(Stewart et al., 2013) | |
| 6.2×10−12 (Δlog k : ± 0.1) | 298 | IUPAC | |
|
| |||
β-pinene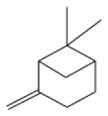
|
(2.36 ± 0.10)×10−12 | 295 | RR/(Atkinson et al., 1984) |
| (2.38 ± 0.05)×10−12 | 296 | RR/(Atkinson et al., 1988) | |
| (1.1 ± 0.4)×10−12 | 298 | RR/(Kotzias et al., 1989) | |
| (2.81 ± 0.47)×10−12 | 298 | RR/(Barnes et al., 1990) | |
| (1.6 ± 1.5)×10−10exp[(−1248 ± 36)/T] | 298–293 | DF-LIF/(Martinez et al., 1998) | |
| (2.1 ± 0.4)×10−12 | 298 | DF-LIF/(Martinez et al., 1998) | |
| (2.81 ± 0.56)×10−12 | 298 | RR/(Kind et al., 1998) | |
| 2.5×10−12 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
Sabinene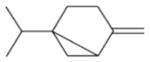
|
(1.01 ± 0.03)×10−11 | 296 | RR/(Atkinson et al., 1990) |
| (1.07 ± 0.16)×10−11 | 298 | DF-LIF/(Martinez et al., 1999) | |
| (2.3 ± 1.3)×10−10exp[(−940 ± 200)/T] | 298–393 | DF-LIF/(Martinez et al., 1999) | |
| 1.0×10−11 (Δlog k : ± 0.15) | 298 | IUPAC | |
|
| |||
Camphene
|
(6.54 ± 0.16)×10−13 | 296 | RR/(Atkinson et al., 1990) |
| (3.1 ± 0.5)×10−12exp[(−481 ± 55)/T] | 298–433 | DF-LIF/(Martinez et al., 1998) | |
| (6.2 ± 2.1)×10−13 | 298 | DF-LIF/(Martinez et al., 1998) | |
|
| |||
2-carene
|
(1.87 ± 0.11)×10−11 | 295 | RR/(Corchnoy and Atkinson, 1990) |
| (2.16 ± 0.36)×10−11 | 295 | RR/(Corchnoy and Atkinson, 1990) | |
| (1.66 ± 0.18)×10−11 | 298 | DF-LIF/(Martínez et al., 1999) | |
| (1.4 ± 0.7)×10−12exp[(741 ± 190)/T] | 298–433 | DF-LIF/(Martínez et al., 1999) | |
| 2.0×10−11 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
3-carene
|
(1.01 ± 0.02)×10−11 | 295 | RR/(Atkinson et al., 1984) |
| (8.2 ± 1.2)×10−11 | 298 | RR/(Barnes et al., 1990) | |
| 9.1×10−11 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
Δ-limonene
|
(1.31 ± 0.04)×10−11 | 295 | RR/(Atkinson et al., 1984) |
| (1.12 ± 0.17)×10−11 | 298 | RR/(Barnes et al., 1990) | |
| (9.4 ± 0.9)×10−12 | 298 | DF-LIF/(Martínez et al., 1999) | |
| 1.2×10−11 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
α-phellandrene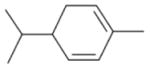
|
(8.52 ± 0.63)×10−11 | 294 | RR/(Atkinson et al., 1985) |
| (5.98 ± 0.20)×10−11 | 298 | RR/(Berndt et al., 1996) | |
| (4.2 ± 1.0)×10−11 | 298 | DF-LIF/(Martínez et al., 1999) | |
| (1.9 ± 1.3)×10−9exp[−(1158 ± 270)/T] | 298–433 | DF-LIF/(Martínez et al., 1999) | |
| 7.3×10−11 (Δlog k : ± 0.15) | 298 | IUPAC | |
|
| |||
β-phellandrene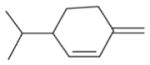
|
(7.96 ± 2.82)×10−12 | 297 | RR/(Shorees et al., 1991) |
|
| |||
α-terpinene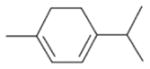
|
(1.82 ± 0.07)×10−10 | 294 | RR/(Atkinson et al., 1985) |
| (1.03 ± 0.06)×10−10 | 298 | RR/(Berndt et al., 1996) | |
| 1.8×10−10 (Δlog k : ± 0.25) | 298 | IUPAC | |
|
| |||
γ-terpinene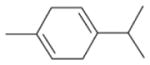
|
(2.94 ± 0.05)×10−11 | 294 | RR/(Atkinson et al., 1985) |
| (2.4 ± 0.7)×10−11 | 298 | DF-LIF/(Martínez et al., 1999) | |
| 2.9×10−11 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
Terpinolene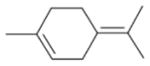
|
(9.67 ± 0.51)×10−11 | 295 | RR/(Corchnoy and Atkinson, 1990) |
| (5.2 ± 0.9)×10−11 | 298 | DF-LIF/(Martínez et al., 1999) | |
| (6.12 ± 0.52)×10−11 | 298 | RR/(Stewart et al., 2013) | |
| 9.7×10−11 (Δlog k : ± 0.25) | 298 | IUPAC | |
|
| |||
Ocimene (cis, trans)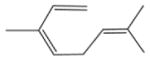
|
(2.23 ± 0.06)×10−11 | 294 | RR/(Atkinson et al., 1985) |
| 2.2×10−11 (Δlog k : ± 0.15) | 298 | IUPAC | |
|
| |||
Myrcene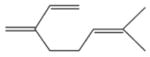
|
(1.06 ± 0.02)×10−11 | 294 | RR/(Atkinson et al., 1985) |
| (1.28 ± 0.11)×10−11 | 298 | DF-LIF/(Martínez et al., 1999) | |
| (2.2± 0.2)×10−12exp[(523 ± 35)/T] | 298–433 | DF-LIF/(Martínez et al., 1999) | |
| 1.1×10−11 (Δlog k : ± 0.12) | 298 | IUPAC | |
|
| |||
α-cedrene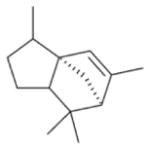
|
(0.82 ± 0.30)×10−11 | 296 | RR/(Shu and Atkinson, 1995) |
|
| |||
α-copaene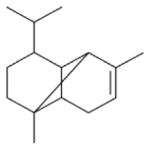
|
(1.6 ± 0.6)×10−11 | 296 | RR/(Shu and Atkinson, 1995) |
|
| |||
β-caryophyllene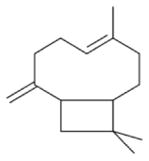
|
(1.9 ± 0.8)×10−11 | 296 | RR/(Shu and Atkinson, 1995) |
|
| |||
α-humulene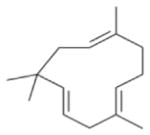
|
(3.5 ± 1.3)×10−11 | 296 | RR/(Shu and Atkinson, 1995) |
|
| |||
Longifolene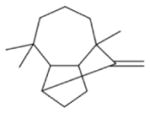
|
(6.8 ± 2.1)×10−13 | 296 | RR/(Shu and Atkinson, 1995) |
|
| |||
Isolongifolene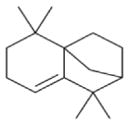
|
(3.9 ± 1.6)×10−12 | 298 | RR/(Canosa-Mas et al., 1999b) |
|
| |||
Alloisolongifolene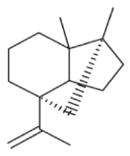
|
(1.4 ± 0.7)×10−12 | 298 | RR/(Canosa-Mas et al., 1999b) |
|
| |||
α-neoclovene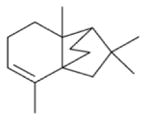
|
(8.2 ± 4.6)×10−12 | 298 | RR/(Canosa-Mas et al., 1999b) |
|
| |||
2-methyl-3-buten-2-ol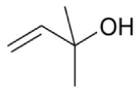
|
4.6×10−14exp[−(400 ± 35)/T] | 267–400 | F-A/(Rudich et al., 1996) |
| (1.21 ± 0.09)×10−14 | 298 | F-A/(Rudich et al., 1996) | |
| (2.1 ± 0.3)×10−14 | 294 | DF-A/(Hallquist et al., 1996) | |
| (1.55 ± 0.55)×10−14 | 294 | RR/(Hallquist et al., 1996) | |
| (8.7 ± 3.0)×10−14 | 298 | RR/(Fantechi et al., 1998b) | |
| (1.0 ± 0.2)×10−14 | 297 | RR/(Noda et al., 2002) | |
| (1.1 ± 0.1)×10−14 | 297 | RR/(Noda et al., 2002) | |
| 1.2×10−14 (Δlog k : ± 0.2) | 298 | IUPAC | |
|
| |||
3-methyl-2-buten-1-ol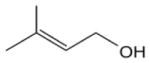
|
(1.0 ± 0.1)×10−12 | 297 | RR/(Noda et al., 2002) |
|
| |||
3-methyl-3-buten-1-ol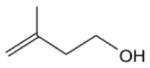
|
(2.7 ± 0.2)×10−13 | 297 | RR/(Noda et al., 2002) |
|
| |||
|
cis-3-hexen-1-ol |
(2.72 ± 0.83)×10−13 | 296 | RR/(Atkinson et al., 1995) |
| (2.67 ± 0.42)×10−13 | 298 | DF-CEAS/(Pfrang et al., 2006) | |
|
| |||
|
trans-3-hexen-1-ol |
(4.43 ± 0.91)×10−13 | 298 | DF-CEAS/(Pfrang et al., 2006) |
|
| |||
cis-4-hexen-1-ol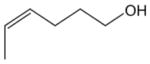
|
(2.93 ± 0.48)×10−13 | 298 | DF-CEAS/(Pfrang et al., 2006) |
|
| |||
|
trans-2-hexen-1-ol |
(1.30 ± 0.24)×10−13 | 298 | DF-CEAS/(Pfrang et al., 2006) |
|
| |||
|
cis-2-hexen-1-ol |
(1.56 ± 0.24)×10−13 | 298 | DF-CEAS/(Pfrang et al., 2006) |
|
| |||
|
trans-2-hexenal |
(1.21 ± 0.44)×10−14 | 296 | RR/(Atkinson et al., 1995) |
| (1.36 ± 0.29)×10−14) | 295 | RR/(Zhao et al., 2011b) | |
| (4.7 ± 1.5)×10−15 | 294 | AR/(Kerdouci et al., 2012) | |
|
| |||
| 4-methylenehex-5-enal |
(4.75 ± 0.35)×10−13 | 296 | RR/(Baker et al., 2004) |
|
| |||
| (3Z)-4-methylhexa-3,5-dienal |
(2.17 ± 0.30)×10−12 | 296 | RR/(Baker et al., 2004) |
|
| |||
(3E)-4-methylhexa-3,5-dienal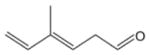
|
(1.75 ± 0.27)×10−12 | 296 | RR/(Baker et al., 2004) |
|
| |||
4-methylcyclohex-3-en-1-one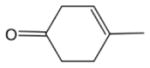
|
(1.81 ± 0.35)×10−12 | 296 | RR/(Baker et al., 2004) |
|
| |||
|
cis-3-hexenyl acetate |
(2.46 ± 0.75)×10−13 | 296 | RR/(Atkinson et al., 1995) |
|
| |||
Methyl vinyl ketone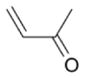
|
< 1.2×10−16 | 298 | F-A/(Rudich et al., 1996) |
| < 6×10−16 | 296 | DF- RR/(Kwok et al., 1996) | |
| (3.2 ± 0.6)×10−16 | 296 | LIF/(Canosa-Mas et al., 1999a) | |
| (5.0 ± 1.2)×10−16 | 296 | RR/(Canosa-Mas et al., 1999a) | |
| < 6×10−16 | 298 | IUPAC | |
|
| |||
Methacrolein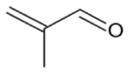
|
(4.46 ± 0.58)×10−15 | 296 | RR/(Kwok et al., 1996) |
| (3.08 ± 0.18)×10−15 | 298 | RR/(Chew et al., 1998) | |
| (3.50 ± 0.15)×10−15 | 298 | RR/(Chew et al., 1998) | |
| (3.72 ± 0.47)×10−15 | 296 | RR/(Canosa-Mas et al., 1999a) | |
| 3.4×10−15 (Δlog k : ± 0.15) | 298 | IUPAC | |
|
| |||
Pinonaldehyde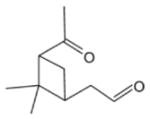
|
(2.40 ± 0.38)×10−14 | 299 | RR/(Hallquist et al., 1997a) |
| (6.0 ± 2.0)×10−14 | 300 | RR/(Glasius et al., 1997) | |
| (2.0 ± 0.9)×10−14 | 296 | RR/(Alvarado et al., 1998) | |
| 2.0×10−14 (Δlog k : ± 0.25) | 298 | IUPAC | |
|
| |||
Linalool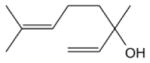
|
(1.12 ± 0.40)×10−11 | 296 | RR/(Atkinson et al., 1995) |
|
| |||
α-terpineol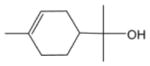
|
(1.6 ± 0.4)×10−11 | 297 | RR/(Jones and Ham, 2008) |
|
| |||
Sabinaketone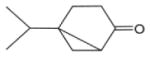
|
(3.6 ± 2.3)×10−16 | 296 | RR/(Alvarado et al., 1998) |
|
| |||
Caronaldehyde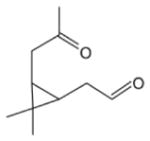
|
(2.5 ± 1.1)×10−14 | 296 | RR/(Alvarado et al., 1998) |
Given uncertainties are those provided by the authors of the kinetic studies. The procedures used to calculate them are not detailed here, as they often differ from one study to another. Readers are referred to the original papers for more information on the uncertainties’ determination.
RR: relative rate; DF-MS: discharge flow–mass spectrometry; DF-LIF: discharge flow–laser–induced fluorescence; DF-A: discharge flow–absorption; DF-CEAS: discharge flow–cavity–enhanced absorption spectroscopy; F-LIF: flow system–laser–induced fluorescence; F-CIMS: flow system–chemical ionization mass spectrometry; F-A: flow system–absorption; PR-A: pulse radiolysis–absorption; AR: absolute rate in simulation chamber.
