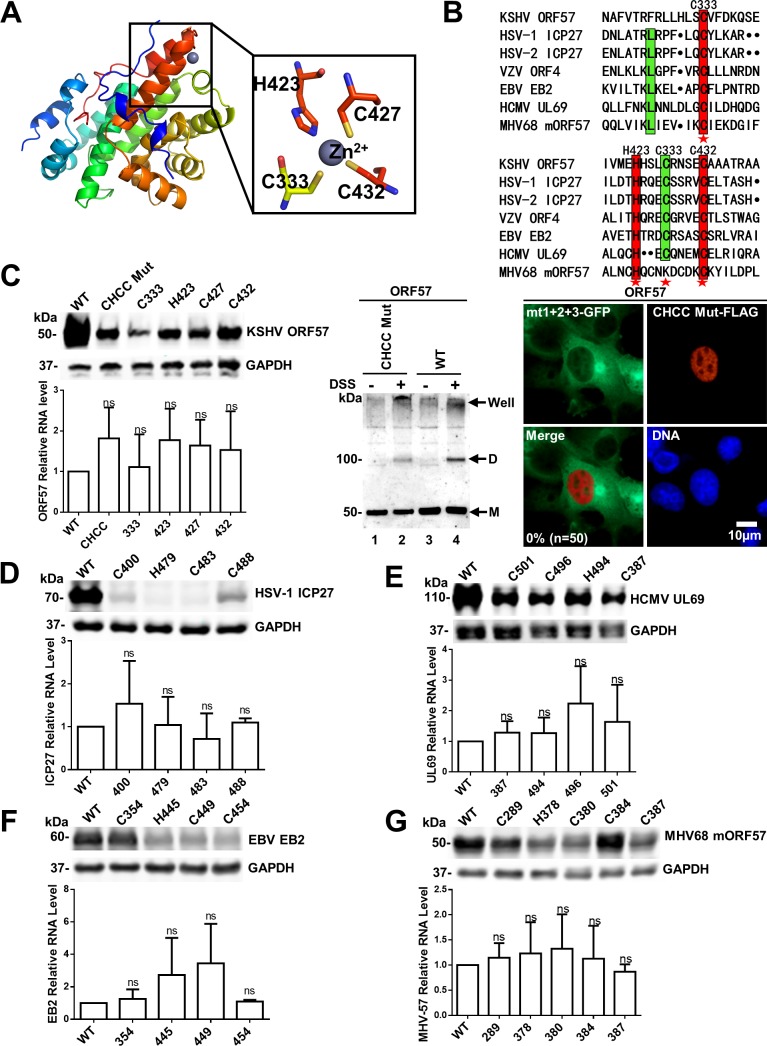
Fig 6. The zinc-binding motif is important for accumulation of KSHV ORF57 protein and its herpesvirus homologues.
(A) The structure of ORF57-CTD in a cartoon model with rainbow spectrum. The zinc (Zn2+) ion (grey sphere) and the surrounding cysteine (C333, C427, C432) and histidine residues (H423) are highlighted and displayed as sticks.(B) Multiple alignment of the protein sequences was performed by Clustal Omega, including the C-terminal domain of KSHV-ORF57, ICP27 (herpes simplex virus type 1 and type 2, HSV1-ICP27 and HSV2-ICP27), ORF4 (varicella-zoster virus, VZV-ORF4), EB2 (Epstein-Barr virus, EBV-EB2), UL69 (human cytomegalovirus, HCMV-UL69), and mORF57 (murine gamma herpesvirus 68, MHV68-mORF57). Conserved residues are labeled in red, identical residues are highlighted in green, and the residues of the zinc-binding motif are marked by red stars. (C) Disruption of the zinc-binding motif reduces the stability and dimerizationin of KSHV ORF57 protein. HEK293 cells were transfected with KSHV ORF57 expression vectors with indicated serine for cysteine and leucine for histidine substitutions in the zinc-binding motif and harvested at 40 h post transfection. The cells were separated into two parts: one for Western blotting (top left panel) and one for RNA analysis (lower left bar graph). WT, wild type; CHCC Mut, a combined serine and leucine substitutions of all four residues in the zinc-binding motif. ORF57 with combined mutations of all four zinc-binding residues was tested for its dimerization activities both in vitro crosslinking and nuclear translocation assays (right two panels) as described in Fig 5D and 5E. M, monomer; D, dimer. (D-G) The zinc-binding motifs in other herpesvirus homologues are important for their protein stability. The similar serine and leucine substitution experiments were conducted to analyze the protein stability of HSV-1 ICP27 (D), HCMV UL69 (E), EBV EB2 (F) and MHV68-mORF57 (G). Results (mean ± SD) are representative of three independent experiments. NS, not significant.