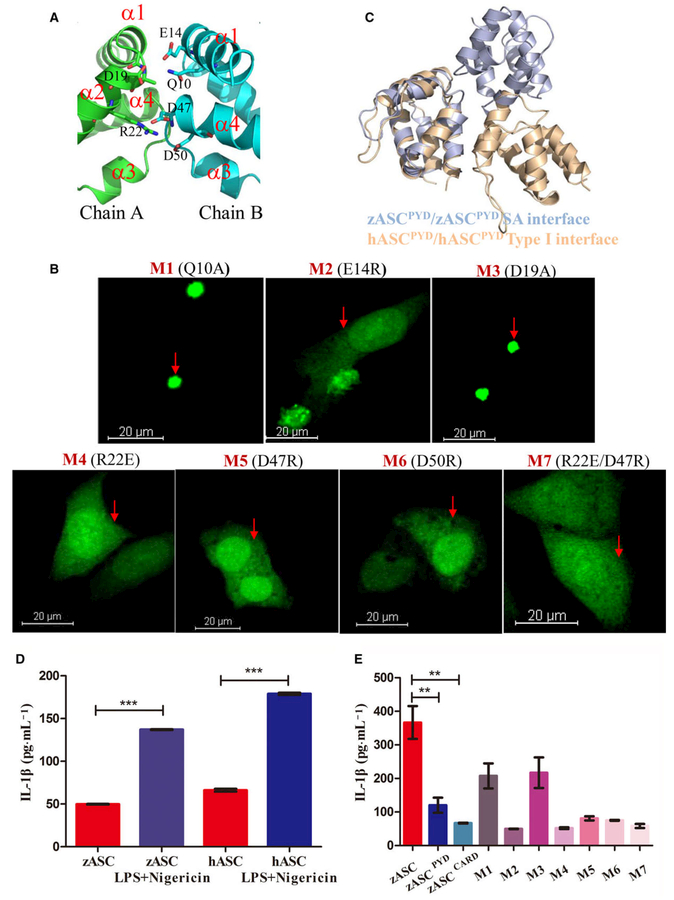
Fig. 4.
Structure-guided mutagenesis of ASC impairs speck formation and inflammasome activity. (A) Detailed interactions in the SA interface. Critical residues in the PYD–PYD interaction interface are shown as stick models and are labeled. Only one half of the symmetric interface is shown for clarity, in which D19 and R22 of chain A (in green) interact with Q10, E14, D47 and D50 of chain B (cyan). (B) Representative images of HeLa cells after ASC mutant overexpression, including Q10A, E14R, D19A, R22E, D47R and D50R. The nucleus was stained with DAPI, and ASC fused with AcGFP oligomerized as a speck. Scale bar: 20 μm. (C) Comparison of the zASCPYD symmetric interface with the conserved type I interface in mammalian ASCPYD. Zebrafish ASC is colored light blue, while human ASC is colored light brown. (D) Reconstitution of the inflammasome in RAW cells transfected with human ASC and zebrafish ASC, in the absence and in the presence of LPS/Negericin treatment (mean ± SEM of at least two experiments). A t test was used for comparison of LPS/negericin treatment with no-treatment group for statistical analysis. ***P < 0.001. (E) Reconstitution of the inflammasome in RAW cells transfected with zASC, zASCPYD, zASCCARD and zASC mutants. LPS-primed RAW264.7 cells were treated with 10 μM nigericin for additional 2 h (mean ± SEM of at least three experiments). A t test is used for comparison of ASC with ASCPYD group and ASC with ASCCARD group for statistical analysis. **P < 0.01, n = 3. M1, mutant 1 (Q10A); M2, mutant 2 (E14R); M3, mutant 3 (D19A); M4, mutant 4 (R22E); M5, mutant 5 (D47R); M6, mutant 6 (D50R); M7, mutant 7 (R22E/D47R).