Figure 6. Virus primed CD4+Tbet+Foxp3− cells upregulate Foxp3 under iTreg and pTreg conditions.
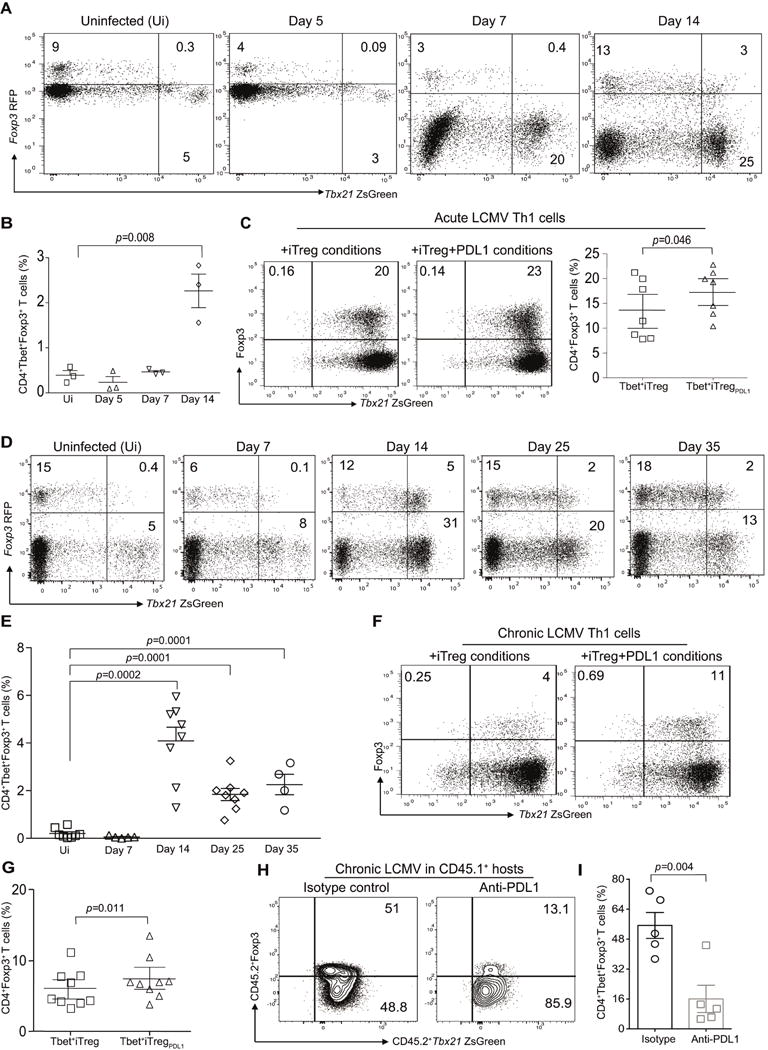
B6.Tbx21ZsgreenFoxp3RFP mice were infected with LCMV Armstrong (2×105 PFU) and then monitored for the presence of CD4+TbethiFoxp3+ cells. Representative flow plots from three individual experiments showing TbethiFoxp3+ cells in the CD4 compartment of murine recipients at various time points post infection (A). Summary of frequency of TbethiFoxp3+ T cells at various time points (B). Virus primed TbethiFoxp3− cells from acute LCMV infected mice were flow sorted at day 14 and then expanded under iTreg conditions in the absence or presence of PDL-1fc chimera. The frequency of Foxp3 expression in TbethiFoxp3− cell population in the absence or presence of PDL-1 after 7 days of ex vivo culture (C). B6.Tbx21ZsgreenFoxp3RFP mice were infected with LCMV clone 13 (2×106 PFU) and then monitored for the presence of CD4+TbethiFoxp3+ cells. Representative flow plots showing TbethiFoxp3+ cells in the CD4 compartment of murine recipients at various time points post infection (D). Summary of frequency of TbethiFoxp3+ T cells at various time points (E). The frequency of Foxp3 expression in TbethiFoxp3− cell population in the absence or presence of PDL-1 after 7 days of ex vivo culture (F). Summary of Tbet+iTreg cell differentiation (G). CD45.2+Tbet+FoxP3− cells (0.7×106 cells) were adoptively transferred into CD45.1+ murine hosts that were infected with Clone-13. Cohorts were treated with either isotype control or anti-PDL1 antibody (200μg/mouse). Splenocytes were harvested at day 10 and the frequency of CD4+Tbet+Foxp3+ pTreg cells were evaluated (H–I). Data are shown as Mean±SEM from a representative of one to three individual experiments involving n=3-9 mice per cohorts. Please also refer to Figure S7.
