Abstract
Background
We aimed to estimate whether elevated levels of complement C3a and C5a in amniotic fluid (AF) are independently associated with increased risks of intra-amniotic infection and/or inflammation (IAI) and spontaneous preterm delivery (SPTD) in women with cervical insufficiency or a short cervix (≤ 25 mm).
Methods
We conducted a retrospective cohort study of 96 consecutive women with cervical insufficiency (n = 62) or a short cervix (n = 34) at 17 to 27 weeks, and who underwent an amniocentesis. AF was cultured and analyzed for C3a and C5a by enzyme-linked immunosorbent assay kits. The primary outcome measures were IAI (defined as a positive AF culture and/or an elevated AF interleukin-6 level [≥ 7.6 ng/mL]) and SPTD at < 32 weeks.
Results
In multivariable analysis, AF level of C3a was the only variable significantly associated with IAI, whereas C5a level in AF and serum C-reactive protein level were not associated with IAI. Using SPTD at < 32 weeks as the outcome variable in logistic regression, elevated AF levels of C3a were associated with increased risk of SPTD at < 32 weeks after adjusting for other baseline confounders, whereas elevated AF levels of C5a were not.
Conclusion
In women with cervical insufficiency or a short cervix, elevated AF level of C3a, but not C5a, is independently associated with increased risks of IAI and SPTD at < 32 weeks. These findings suggest that subclinical IAI or SPTD in the context of cervical insufficiency is related to activation of complement system in AF.
Keywords: Amniotic Fluid; Complement C3a, C5a; Cervical Insufficiency; Intra-amniotic Infection; Preterm Delivery
Graphical Abstract
INTRODUCTION
Cervical insufficiency and a mid-trimester short cervix, which may be understood to occur along a continuum of severity, complicate approximately 0.1%–2% of all pregnancies1,2 and 0.5%–3% of asymptomatic singleton pregnancies,3,4 and are important causes of spontaneous preterm delivery (SPTD).5 Although the causes for cervical insufficiency/short cervix and for the consequent SPTD may be multiple, intra-uterine infection/inflammation and premature cervical change are thought to be the major potential mechanisms underlying these context-dependent causes.5,6,7 However, little is known about the possible biomarkers, opening a window into understanding these biological mechanisms and those involved in disease etiologies, which lead to the development of more effective prevention and treatment strategies for SPTD.
In 1899, Bordet reported that activation of the complement system, which plays a central role in innate immunity, generates anaphylatoxins (C3a, C4a, and C5a). It is well known that the anaphylatoxins function as a bridge between the innate and adaptive immunity and are responsible for controlling the local pro-inflammatory response through various mechanisms, including activation of leukocytes and chemotaxis.8,9 Subsequently, several studies have focused on the relationship between the anaphylatoxins, immunoinflammatory disease, and complications of pregnancy (e.g., SPTD, preeclampsia, spontaneous fetal loss), and have shown significant associations with the disease.8 In particular, increased anaphylatoxins generated by activation of the complement system in the amniotic fluid (AF; C3a, C4a, and C5a) and maternal blood (C5a) have been reported to be associated with intra-amniotic infection in preterm labor in several publications.10,11,12 Importantly, in a mouse model of infection-induced preterm labor, Gonzalez et al.13,14 have shown that complement activation in serum plays a causative role in cervical remodeling that leads to preterm delivery, suggesting that the increase in complement activation fragments may be related to cervical dilatation and effacement. However, to date, there is no information regarding activation of the complement system in AF in women with cervical insufficiency/short cervix in relation to intra-amniotic infection and/or inflammation (IAI) and SPTD. This study aimed to determine whether elevated levels of complement C3a and C5a in AF are independently associated with an increased risk of IAI and SPTD in women with cervical insufficiency or a short cervix (≤ 25 mm).
METHODS
Study design
A retrospective study was conducted from September 2004 through September 2014 at Seoul National University Bundang Hospital (Seongnam, Korea). The study population consisted of all pregnant women with singleton pregnancies diagnosed with cervical insufficiency or a short cervix (≤ 25 mm) at 17 + 0 and 27 + 6 weeks of gestation, who underwent amniocentesis. Women with the following conditions were excluded: a dead fetus, preterm labor, or preterm premature rupture of the membranes at diagnosis, prior cervical cerclage, multiple pregnancy, clinical signs of chorioamnionitis, unavailable outcome data, a missing AF sample, and major fetal congenital anomalies. Gestational age was calculated based on the last menstrual period, and first trimester or second trimester (≤ 20 weeks) ultrasound results, when available. The primary outcome measures were IAI and SPTD at < 32 weeks of gestation.
AF sample and analysis of complement C3a and C5a levels in the AF
At the time of admission, AF samples were obtained from all participants via transabdominal amniocentesis to evaluate the microbiologic and inflammatory status of the amniotic cavity and/or to reduce tension in the amniotic cavity and allow for retraction prior to cerclage placement. The retrieved AF was sent to the laboratory for culture of aerobic/anaerobic bacteria and genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis), the methods for which have previously been described in detail elsewhere.15 The remaining AF was centrifuged at 1,500 × g at 4°C for 10 minutes, and the supernatant was aliquoted and stored at −70°C until complement analysis was completed. Enzyme-linked immunosorbent assay (ELISA) kits were used to measure interleukin (IL)-6 (DuoSet ELISA; R&D Systems, Minneapolis, MN, USA) and complement C3a and C5a (BD Biosciences, San Diego, CA, USA) in the AF samples, according to the manufacturer's instructions. The ranges of the IL-6, C3a, and C5a standard curves were 7.8–600 pg/mL, 0.078–2.5 ng/mL, and 0.08–2.5 ng/mL, respectively. All the samples were assayed in duplicate. The intra- and inter-assay coefficients of variation were < 10% each. C-reactive protein (CRP) in maternal blood was usually measured within 2 to 3 hours of amniocentesis.
Definitions of various factors and management of cervical insufficiency and/or a short cervix
Intra-amniotic infection was defined as a positive AF culture for microorganisms; intra-amniotic inflammation was defined as elevated AF concentrations of IL-6 (IL-6 ≥ 7.6 ng/mL), as previously reported by our group in cervical insufficiency/a short cervix.16 IAI was defined as a positive AF culture and/or the presence of intra-amniotic inflammation. Cervical insufficiency was defined as a painless cervical dilation ≥ 1 cm and bulging of the fetal membranes into a widened internal os determined via visual evaluation using a sterile speculum, in the absence of contractions and labor. A short cervix was defined as a cervical length of ≤ 25 mm on transvaginal sonographic assessment. Cervical length was measured using the standard technique, as previously described in detail elsewhere.17 Women with suspected cervical insufficiency and visible fetal membranes were considered as a nonmeasurable cervical length (described as 0-mm cervix). The detailed description of our management of cervical insufficiency or a short cervix has been previously published elsewhere.16,18 In brief, the decisions on the placement of a cervical cerclage, progesterone supplementation, and antibiotic and tocolytic treatments were left to the discretion of the attending obstetrician. Antenatal corticosteroids were administered between 24 and 34 weeks of gestation. Medications such as antibiotics, corticosteroids, and tocolytics were started after amniocentesis. A histologic diagnosis of chorioamnionitis was made in accordance with the definition previously described in detail.19 Clinical chorioamnionitis was diagnosed in accordance with the criteria proposed by Gibbs et al.20
Statistical methods
The Shapiro-Wilk test was used to assess whether data in groups were normally distributed. Continuous data were analyzed using the Student's t-test or Mann-Whitney U test, while categorical data were compared using the χ2 test or Fisher's exact test, as appropriate. A multivariate logistic regression analysis was then performed to determine the independent relationship between complement C3 and C5a levels in AF and the primary outcome measures after adjusting for potential baseline variables showing a significant association with these outcomes in univariate analysis (P < 0.05). In multivariable analyses for outcome of SPTD at < 32 weeks in which the ratio of the number of events per independent variable analyzed is too small, the validity of the logistic model could be questionable.21 Therefore, we first attempted to determine the candidate independent variables included in the final regression model using a backward stepwise selection, with a P value of < 0.05 as a criterion for model inclusion; thereafter, these independent variables were evaluated in a full logistic regression model.22 Continuous variables were examined for linearity in the logit by using link test.23 The Spearman rank correlation test was used to measure the relationship between the continuous variables that did not follow a normal distribution. All statistical analyses were performed using a two-sided test, with a significance level of 0.05. Statistical analyses were performed using the SPSS version 22.0 for Windows (IBM SPSS Statistics, Chicago, IL, USA).
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-1311/228-010). The patients provided written informed consent for the amniocentesis procedure and use of the AF samples for research purposes.
RESULTS
During the study period, 96 women with cervical insufficiency (n = 66) or an asymptomatic short cervix (n = 30) met the eligibility criteria and were included in the final analysis. Their mean gestational age at the time of sampling was 22.0 (standard deviation, 2.3 weeks; range, 17.1–27.5) weeks. The prevalence of positive AF culture and intra-amniotic inflammation was 9.4% (8/96) and 29.2% (28/96), respectively. Intra-amniotic inflammation was present in 75% (6 of 8) of the women with a positive AF culture and in 25.6% (22 of 86) of women with a negative AF culture. The microorganisms isolated from the amniotic cavity were U. urealyticum (n = 8) and M. hominis (n = 4). Polymicrobial invasion was detected in 4 out of 8 women (50%). SPTD at < 32 weeks of gestation occurred in 45.8% (44/96) of women.
The levels of AF C3a, C5a, and IL-6 were significantly correlated with each other (all variables, r = 0.225–0.430; P < 0.05). Cervical dilatation was significantly correlated with AF IL-6 (r = 0.406; P < 0.001) but not with the levels of AF C3a (r = 0.101; P = 0.331) or C5a (r = 0.108; P = 0.299). Similarly, cervical length was also significantly correlated with AF IL-6 (r = −0.426; P < 0.001) but not with the levels of AF C3a (r = −0.164; P = 0.111) or C5a (r = −0.181; P = 0.078). However, there was no correlation between the C3a, C5a, and IL-6 levels measured in the AF and gestational age at sampling.
Table 1 shows the demographic and clinical characteristics of the women according to the presence or absence of IAI. Compared to women without IAI, those with IAI had a significantly higher mean serum CRP level, more advanced cervical dilatation, and a lower mean gestational age at delivery. Moreover, the mean AF levels of C3a, but not C5a, were significantly higher in women with IAI than in those without IAI. However, there were no significant associations between IAI and maternal age, nulliparity, prior spontaneous preterm birth, disease entity, and cervical length and gestational age at the time of amniocentesis.
Table 1. Demographic and clinical characteristics of the study population according to the presence or absence of IAI.
| Variables | IAI | P value | ||
|---|---|---|---|---|
| Negative (n = 66) | Positive (n = 30) | |||
| Maternal age, yr | 32.1 ± 3.5 | 31.1 ± 2.6 | 0.141 | |
| Nulliparity | 47.0 (31) | 43.3 (13) | 0.740 | |
| Prior spontaneous preterm birth, < 37 wk | 13.6 (9) | 6.7 (2) | 0.494 | |
| Disease entity | 0.227 | |||
| Cervical insufficiency | 60.6 (40) | 73.3 (22) | ||
| Short cervix | 39.4 (26) | 26.7 (8) | ||
| Gestational age at amniocentesis, wk | 22.2 ± 2.3 | 21.5 ± 2.2 | 0.530 | |
| Gestational age at delivery, wk | 32.9 ± 6.8 | 27.5 ± 6.3 | 0.001 | |
| Cervical length by ultrasound, mm | 4.8 ± 7.1 | 2.8 ± 5.6 | 0.148 | |
| Cervical dilatation, cm | 1.2 (0–8) | 3.0 (0–6) | 0.029 | |
| > 2 | 22.7 (15) | 53.3 (16) | 0.003 | |
| ≤ 2 | 77.3 (51) | 46.7 (14) | ||
| Serum CRP, mg/L | 6.9 ± 10.9 | 12.9 ± 13.4 | 0.005 | |
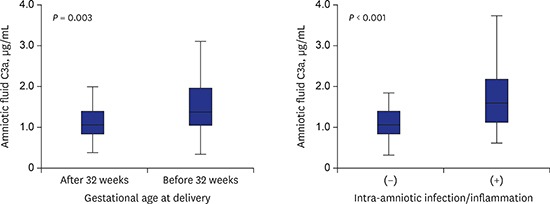
| AF C3a levels, µg/mL | 1.17 ± 0.49 | 1.76 ± 0.79 | < 0.001 | |
| AF C5a levels, ng/mL | 6.21 ± 4.95 | 8.42 ± 6.90 | 0.109 | |
| Use of tocolytics | 42.4 (28) | 53.3 (16) | 0.323 | |
| Use of corticosteroids | 33.3 (22) | 36.7 (11) | 0.751 | |
| Use of antibiotics | 87.9 (58) | 93.3 (28) | 0.720 | |
| Clinical chorioamnionitis | 4.5 (3) | 3.3 (1) | 1.000 | |
| Histologic chorioamnionitisa | 45.5 (15/33) | 74.1 (20/27) | 0.025 | |
Data are presented as means ± standard deviation, the median (range) or % (number).
IAI = intra-amniotic infection and/or inflammation, CRP = C-reactive protein, AF = amniotic fluid.
aData for the histologic evaluation of the placenta were available in 60 (62.5%) of the 96 women because in 5 cases, the delivery took place at another institution and in 31 cases, histologic evaluation of the placenta was not performed because of our institutional policy that only the placentas in cases of preterm delivery are to be sent for histopathologic examination.
Table 2 presents the clinical and laboratory characteristics of the study population stratified according to SPTD at < 32 weeks of gestation. Compared to women who did not deliver spontaneously at < 32 weeks, women who had SPTD at < 32 weeks had a significantly more advanced cervical dilatation, lower mean cervical length at presentation, higher proportion of cervical insufficiency and IAI, a higher rate of administration of tocolytics and antibiotics, and were less likely to receive progesterone therapy and cerclage placement. The mean AF levels of C3a and C5a were significantly higher in women who had SPTD at < 32 weeks than in those who did not deliver spontaneously at < 32 weeks. However, there were no significant differences on maternal age, parity, prior spontaneous preterm birth, gestational age at sampling, serum CRP levels, and use of corticosteroids between women who had SPTD at < 32 weeks and those who delivered at ≥ 32 weeks.
Table 2. Demographic and clinical characteristics of the study population stratified according to SPTD at < 32 weeks.
| Variables | Delivery < 32 wk (n = 44) | Delivery ≥ 32 wk (n = 52) | P value | |
|---|---|---|---|---|
| Maternal age, yr | 31.3 ± 2.7 | 32.1 ± 3.6 | 0.222 | |
| Nulliparity | 54.5 (24) | 38.5 (20) | 0.117 | |
| Prior spontaneous preterm birth, < 37 wk | 13.4 (5) | 11.5 (6) | 0.979 | |
| Gestational age at amniocentesis, wk | 21.7 ± 2.4 | 22.2 ± 2.1 | 0.478 | |
| Disease entity | 0.001 | |||
| Cervical insufficiency | 81.8 (36) | 50.0 (26) | ||
| Short cervix | 18.2 (8) | 50.0 (26) | ||
| Cervical length by ultrasound, mm | 2.0 ± 4.9 | 5.9 ± 7.5 | 0.002 | |
| Cervical dilatation, cm | 3 (0–8) | 0.5 (0–8) | < 0.001 | |
| > 2 | 52.3 (23) | 15.4 (8) | < 0.001 | |
| ≤ 2 | 47.7 (21) | 84.6 (44) | ||
| Serum CRP, mg/L | 12.8 ± 15.9 | 5.5 ± 5.6 | 0.127 | |
| Vaginal progesterone therapy | 11.4 (5) | 32.7 (17) | 0.013 | |
| Rescue cerclage | 54.5 (24) | 78.8 (41) | 0.011 | |
| Use of tocolytics | 59.1 (26) | 34.6 (18) | 0.016 | |
| Use of corticosteroids | 43.2 (19) | 26.9 (14) | 0.095 | |
| Use of antibiotics | 97.7 (43) | 82.7 (43) | 0.019 | |
| Clinical chorioamnionitis | 9.1 (4) | 0 (0) | 0.027 | |
| Histologic chorioamnionitisa | 73.8 (31/42) | 22.2 (4/18) | < 0.001 | |
| IAI | 47.7 (21) | 17.3 (9) | 0.001 | |
| Intra-amniotic inflammation | 45.5 (20) | 15.4 (8) | 0.001 | |
| Positive AF cultures | 14.0 (6/43) | 3.9 (2/51) | 0.084 | |
| AF C3a levels, µg/mL | 1.59 ± 0.78 | 1.16 ± 0.46 | 0.003 | |
| AF C5a levels, ng/mL | 7.96 ± 6.39 | 6.01 ± 4.91 | 0.044 | |
| Gestational age at delivery, wk | 24.2 ± 3.7 | 37.1 ± 2.0 | < 0.001 | |
Data are presented as means ± standard deviation, the median (range) or % (number).
SPTD = spontaneous preterm delivery, CRP = C-reactive protein, IAI = intra-amniotic infection and/or inflammation, AF = amniotic fluid.
aData for the histologic evaluation of the placenta were available in 60 (62.5%) of the 96 women because in 5 cases, the delivery took place at another institution and in 31 cases, histologic evaluation of the placenta was not performed because of our institutional policy that only the placentas in cases of preterm delivery are to be sent for histopathologic examination.
As shown in Table 3, elevated AF level of C3a was significantly associated with an increased risk of IAI even after adjustment for confounders (cervical dilatation and CRP). When all variables with P values of < 0.05 from the univariate analysis were included in the multivariate analysis, the multivariate analysis with SPTD as a dependent variable included 9 independent variables with 44 events in the cohort, with a low event per variable of 4.8. Therefore, we attempted to select candidate variables based on the results of stepwise backward analysis for the primary endpoint and their clinical importance in the literature. In stepwise backward analysis, cervical dilatation, IAI, rescue cerclage, use of antibiotics, and AF C3a levels had the most significant associations with SPTD at < 32 weeks and were, therefore, included in the full multivariate model (Table 3). In the resulting parsimonious multivariate model, elevated AF level of C3a is associated with increased risk of SPTD at < 32 weeks after adjusting for other baseline confounders. No statistically significant associations were found between elevated AF level of C5a and SPTD at < 32 weeks when AF C5a level was entered into the model instead of AF C3a levels (AF C5a; odds ratio [OR], 1.057; 95% confidence interval [CI], 0.957–1.167; P < 0.272).
Table 3. Relationship of AF C3a and C5a with the risk of IAI and spontaneous preterm birth, analyzed by multivariable logistic regression.
| Risk factors | OR | 95% CI | P value | |
|---|---|---|---|---|
| (A) Dependent variable: IAI | ||||
| Cervical dilatation, cm | 1.289 | 0.976–1.702 | 0.074 | |
| CRP, mg/L | 1.030 | 0.992–1.070 | 0.128 | |
| AF C3a levels, µg/mL | 4.239 | 1.850–9.712 | 0.001 | |
| (B) Dependent variable: SPTD, < 32 wk | ||||
| AF C3a levels, µg/mL | 3.027 | 1.064–8.611 | 0.038 | |
| Cervical dilatation, cm | 1.669 | 1.202–2.317 | 0.002 | |
| IAI | 5.815 | 1.456–23.218 | 0.013 | |
| Rescue cerclage | 0.150 | 0.042–0.539 | 0.004 | |
| Vaginal progesterone therapy | 0.065 | 0.012–0.344 | 0.001 | |
| Use of antibiotics | 63.245 | 1.104–3,624.610 | 0.045 | |
AF = amniotic fluid, IAI = intra-amniotic infection and/or inflammation, OR = odds ratio, CI = confidence interval, CRP = C-reactive protein, SPTD = spontaneous preterm delivery.
Table 4 shows the clinical and laboratory characteristics of the study population according to disease entity. Compared to women with a short cervix, those with cervical insufficiency delivered significantly earlier and had higher risks of preterm delivery before 32 weeks. The median AF levels of IL-6, but not C3a and C5a, were significantly higher in the women with cervical insufficiency than in those with a short cervix.
Table 4. Demographic, clinical, and laboratory characteristics of the study population according to disease entity.
| Variables | Disease entity | P value | |
|---|---|---|---|
| Cervical insufficiency (n = 62) | Short cervix (n = 34) | ||
| Maternal age, yr | 31.5 ± 3.1 | 32.2 ± 3.6 | 0.402 |
| Nulliparity | 50.0 (31) | 38.2 (13) | 0.269 |
| Gestational age at amniocentesis, wk | 21.7 ± 2.2 | 22.4 ± 2.3 | 0.401 |
| Gestational age at delivery, wk | 29.3 ± 7.2 | 34.7 ± 5.3 | 0.001 |
| SPTD, < 32 wk | 58.1 (36) | 23.5 (8) | 0.001 |
| Cerclage placement | 66.1 (41) | 70.6 (24) | 0.655 |
| Vaginal progesterone therapy | 21 (13) | 26.5 (9) | 0.540 |
| Serum CRP, mg/L | 9.8 ± 12.4 | 7.0 ± 11.2 | 0.052 |
| IAI | 35.5 (22) | 23.5 (8) | 0.227 |
| Intra-amniotic inflammation | 35.5 (22) | 17.6 (6) | 0.067 |
| Positive amniotic fluid cultures | 8.1 (5) | 9.4 (3) | 1.000 |
| AF IL-6, ng/mL | 13.95 ± 31.91 | 5.46 ± 13.88 | < 0.001 |
| AF C3a levels, µg/mL | 1.42 ± 0.66 | 1.23 ± 0.64 | 0.061 |
| AF C5a levels, ng/mL | 7.17 ± 5.65 | 6.42 ± 5.82 | 0.174 |
| Use of tocolytics | 50.0 (31) | 38.2 (13) | 0.269 |
| Use of antenatal corticosteroids | 33.9 (21) | 35.3 (12) | 1.000 |
| Use of antibiotics | 98.4 (61) | 73.5 (25) | < 0.001 |
| Clinical chorioamnionitis | 6.5 (4) | 0 (0) | 0.294 |
| Histologic chorioamnionitisa | 64.4 (29/45) | 40.0 (6/15) | 0.099 |
Values are given as means ± standard deviation (range) or % (number).
SPTD = spontaneous preterm delivery, CRP = C-reactive protein, IAI = intra-amniotic infection and/or inflammation, AF = amniotic fluid, IL = interleukin.
aData for the histologic evaluation of the placenta were available in 60 (62.5%) of the 96 women because in 5 cases, the delivery took place at another institution and in 31 cases, histologic evaluation of the placenta was not performed because of our institutional policy that only the placentas in cases of preterm delivery are to be sent for histopathologic examination.
DISCUSSION
The results of the current study clearly demonstrate that in women with cervical insufficiency or a short cervix, elevated AF level of C3a, but not C5a, is independently associated with increased risks of IAI and SPTD at < 32 weeks. These observations suggest that complement activation in AF may play an important role in the regulation of the host response to IAI and in the mechanism of preterm parturition in the context of asymptomatic cervical insufficiency/short cervix. Similar observations have also been reported in the AF and maternal serum of women with preterm labor and intact membranes.10,11,12,24,25
Several studies have addressed the influence of microbial invasion of the amniotic cavity on the AF levels of anaphylatoxins in women with preterm labor and intact membranes. Elimian et al.12 found AF C3 (total protein) to be a predictive factor for intra-amniotic infection. Soto et al.,10 who conducted a cross-sectional study, reported that compared to women with preterm labor with negative AF cultures, those with positive AF cultures had significantly increased median AF levels of complement split products C3a, C4a, and C5a. In accordance with previous studies on women with preterm labor, we also found that an elevated AF level of C3a is independently associated with an increased risk of IAI or both in our population. Taken together, these observations strongly support the participation of C3a in the host immune response against IAI. In fact, these findings are not unexpected, given that 1) during normal pregnancy, the complement system, which comprises a key component of the innate immunity, is found in the AF10,26 and 2) its activation results in an enzymatic cascade, leading to the production of anaphylatoxins responsible for clearing pathogens and generating an inflammatory response through the production of potent pro-inflammatory mediators.27
Interestingly, Lynch et al.28 found that elevated plasma levels of the complement activation fragment C3a in early gestation (< 20 weeks) are an independent predictive factor for SPTD. Similarly, in the AF compartment in asymptomatic women with cervical insufficiency/short cervix, we also found that an elevated AF level of C3a, as measured during mid-trimester, increases the risk of SPTD at < 32 weeks. Further, multivariable analysis demonstrated that this risk is independent of the presence of IAI, which, in the published literature, has been reported to be an important risk factor for SPTD in cervical insufficiency/short cervix.16,29 Consequently, our data and the data of Lynch et al.28 suggest a role for C3a in the pathogenesis of preterm parturition, independent of its critical role of infection/inflammation and, thus, complement might be a selective and specific target for therapy to prevent preterm delivery in women with cervical insufficiency/short cervix. Future studies are required to determine whether a neutralizing antibody against a complement regulatory protein at the C3 convertase level would significantly prolong pregnancy in women with cervical insufficiency/short cervix in the context of clinical trials.
We found that neither AF C3a nor C5a levels correlated with cervical dilatation or cervical length. In addition, the AF concentrations of C3a and C5a were not different between women with cervical insufficiency and those with a short cervix, although there may be a likelihood of a type II error (for C3a, β = 0.737, power 0.263; for C5a, β = 0.907, power 0.093) given the small sample size. These findings are in contrast to the results of previous studies on animal models of infection-induced preterm labor, which have shown that anaphylatoxins (C3a and C5a), as observed in the serum, play a crucial role in the cervical remodeling process that ripens and dilates the cervix in preterm labor but not in term labor in mice.13,14 Also, our unpublished but submitted results on maternal plasma levels of C3a and C5a agree with this data, as plasma C3a and C5a levels were significantly higher in women with cervical insufficiency than in those with a short cervix, and the plasma C3a level was significantly and positively correlated with cervical dilatation.30 In contrast, pro-inflammatory cytokine levels in AF were significantly higher in the group of women with cervical insufficiency than in the group of women with a short cervix, and had significant correlations with cervical dilatation and cervical length, as shown in the current and previous studies.16,18,31 Taken together, these findings suggest that the immune system or inflammatory response may play an important role in the mechanisms that lead to cervical insufficiency, but its patterns are markedly different in AF and plasma compartments. Therefore, investigations into mechanisms by which immune system or inflammatory response can modify cervical ripening and dilatation should take into account that differential immune responses may occur according to compartments (plasma, AF, and cervicovaginal swab).
The present study has several limitations. First, the study population comprised individuals with heterogeneous disease entities, even if a short cervix is not the same disease entity as cervical insufficiency. However, this may not alter our main findings, because we took into account the effect of this confounding variable in the univariate and multivariate analyses. Second, the complement activation fragments measured were not fully characterized for 3 initial mechanisms known as the classical, lectin, and alternative pathways.27,28 Thus, the current study provided no data to determine the complement pathway that is specifically involved in the IAI and SPTD in cases of cervical insufficiency. Third, the study was of a retrospective nature and used a relatively small sample size from a single center, which may limit the generalizability of the study findings. Fourth, not every woman with suspected cervical insufficiency/short cervix who was admitted to our institution underwent amniocentesis. This may cause a concern for a potential selection bias. Fifth, the study subjects were recruited over a period of 10 years in order to collect extensive data despite improvements in medical care (e.g., vaginal progesterone) to prevent preterm births in asymptomatic women during this period. This may modify the impact of various risk factors on SPTD before 32 weeks of gestation. However, it is unlikely that this has altered our main findings because we adjusted for these confounding variables in the multivariate analysis. The strength of the study is that this is the first study, to our knowledge, to examine the association of changes in complement activation fragments present in the AF compartment with IAI and SPTD in women with cervical insufficiency/short cervix.
In conclusion, in women with cervical insufficiency or a short cervix, elevated AF level of C3a, but not C5a, is independently associated with an increased risk of IAI and has association with SPTD, independent of the presence of IAI. Further larger prospective studies are warranted to determine whether AF C3a, independently or in combination with known risk factors (i.e., AF pro-inflammatory cytokines and cervical dilatation), will play an important role in patient selection for cerclage placement.
Footnotes
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant No. NRF-2017R1D1A1B03030826).
Disclosure: The authors have no potential conflict of interest to disclose.
Author Contributions: Conceptualization: Kim YM, Park KH, Yoo HN. Dara curation: Kim YM, Park KH, Yoo HN, Jeon SJ. Formal analysis: Kim YM, Park KH, Kook SY, Park H. Methodology: Kim YM, Park KH, Yoo HN. Writing - original draft: Kim YM, Park KH, Kook SY. Writing - review & editing: Kim YM, Park KH, Kook SY, Park H, Yoo HN, Jeon SJ.
References
- 1.Lidegaard O. Cervical incompetence and cerclage in Denmark 1980–1990. A register based epidemiological survey. Acta Obstet Gynecol Scand. 1994;73(1):35–38. doi: 10.3109/00016349409013390. [DOI] [PubMed] [Google Scholar]
- 2.Harger JH. Cervical cerclage: patient selection, morbidity, and success rates. Clin Perinatol. 1983;10(2):321–341. [PubMed] [Google Scholar]
- 3.Cho SH, Park KH, Jung EY, Joo JK, Jang JA, Yoo HN. Maternal characteristics, short mid-trimester cervical length, and preterm delivery. J Korean Med Sci. 2017;32(3):488–494. doi: 10.3346/jkms.2017.32.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuusela P, Jacobsson B, Söderlund M, Bejlum C, Almström E, Ladfors L, et al. Transvaginal sonographic evaluation of cervical length in the second trimester of asymptomatic singleton pregnancies, and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2015;94(6):598–607. doi: 10.1111/aogs.12622. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194(1):1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham FG, Bloom SL, Spong CY, Dashe JS, Hoffman BL, Casey BM, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw-Hill Education; 2014. Preterm labor; pp. 836–837. [Google Scholar]
- 8.Denny KJ, Woodruff TM, Taylor SM, Callaway LK. Complement in pregnancy: a delicate balance. Am J Reprod Immunol. 2013;69(1):3–11. doi: 10.1111/aji.12000. [DOI] [PubMed] [Google Scholar]
- 9.Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a) Int J Biochem Cell Biol. 2009;41(11):2114–2117. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Soto E, Romero R, Richani K, Yoon BH, Chaiworapongsa T, Vaisbuch E, et al. Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. J Matern Fetal Neonatal Med. 2009;22(11):983–992. doi: 10.3109/14767050902994747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, et al. Anaphylatoxins in preterm and term labor. J Perinat Med. 2005;33(4):306–313. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elimian A, Figueroa R, Canterino J, Verma U, Aguero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92(1):72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179(2):838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PLoS One. 2011;6(11):e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Kim A. Intra-amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci. 2013;28(8):1226–1232. doi: 10.3346/jkms.2013.28.8.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung EY, Park KH, Lee SY, Ryu A, Oh KJ. Non-invasive prediction of intra-amniotic infection and/or inflammation in patients with cervical insufficiency or an asymptomatic short cervix (≤15 mm) Arch Gynecol Obstet. 2015;292(3):579–587. doi: 10.1007/s00404-015-3684-3. [DOI] [PubMed] [Google Scholar]
- 17.Suh YH, Park KH, Hong JS, Noh JH. Prediction of prolonged pregnancy in nulliparous women by transvaginal ultrasonographic measurement of cervical length at 20–24 weeks and 37 weeks. J Korean Med Sci. 2007;22(1):89–93. doi: 10.3346/jkms.2007.22.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung EY, Park KH, Lee SY, Ryu A, Joo JK, Park JW. Predicting outcomes of emergency cerclage in women with cervical insufficiency using inflammatory markers in maternal blood and amniotic fluid. Int J Gynaecol Obstet. 2016;132(2):165–169. doi: 10.1016/j.ijgo.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172(3):960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 22.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 23.Pregibon D. Logistic regression diagnostics. Ann Stat. 1981;9(4):705–724. [Google Scholar]
- 24.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, et al. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63(4):318–330. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22(10):905–916. doi: 10.1080/14767050902994663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stabile I, Grudzinskas JG, Nicolaides KH, Rodeck C, Bach A, Teisner B, et al. Complement factors in fetal and maternal blood and amniotic fluid during the second trimester of normal pregnancy. Br J Obstet Gynaecol. 1988;95(3):281–285. doi: 10.1111/j.1471-0528.1988.tb06870.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 28.Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117(1):75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(6):633.e1–633.e8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Cho SH, Han BR, Oh KJ, Hong JS, Park KH. Maternal plasma C3a and C5a for predicting spontaneous preterm delivery and intra-amniotic infection in women with cervical insufficiency [abstract] Seongnam: Korean Society of Maternal Fetal Medicine; 2015. [Google Scholar]
- 31.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200(4):374.e1–374.e5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]


