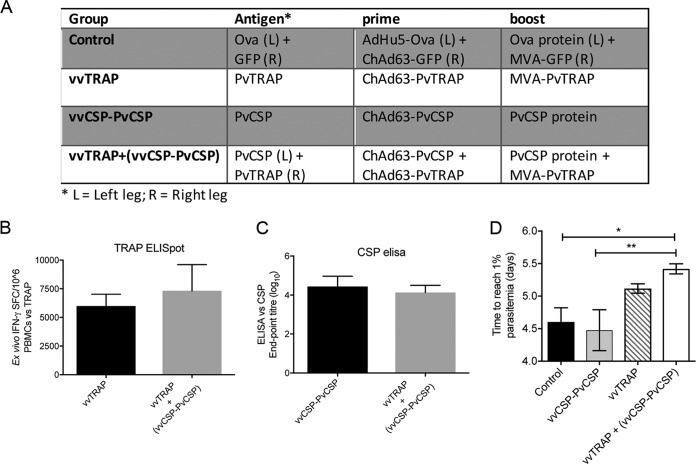
FIG 2.
Immunogenicity and protective efficacy of a combination P. vivax CSP-VK210 with adenoviral or MVA viral vectors expressing P. vivax TRAP (vvTRAP). (A) Inbred female BALB/c mice (n = 7 to 8/group) were primed with PvCSP-VK210 viral vector (vv) followed by the PvCSP protein (vvCSP-PvCSP), ChAd63-MVA viral vector expressing PvTRAP (vvTRAP), or their combination (vvTRAP+vvCSP-PvCSP). (B) Two weeks postboost ex vivo IFN-γ ELISpot responses were measured upon restimulation of whole-blood PBMCs with three subpools of peptides representing the full-length PvTRAP. Values are means and standard errors of the means. (C) An endpoint titer ELISA was done to quantify anti-PvCSP-VK210 antibodies. Bars indicate median responses. (D) Two weeks after the last vaccination, animals were challenged by intravenous injection of 2,000 double-transgenic P. berghei PvCSP-VK210 and PvTRAP sporozoites. Blood stage infection was recorded over three consecutive days starting at day 5 postchallenge, and a linear regression model predicting time to 1% blood stage parasitemia for individual animals was applied, showing a significant delay in the vvTRAP+vvCSP-PvCSP group that received a combination vaccine compared to time for the other treatment groups. Values are means and standard errors of the means. *, P < 0.05; **, P < 0.01 (Mann-Whitney test comparing two groups).