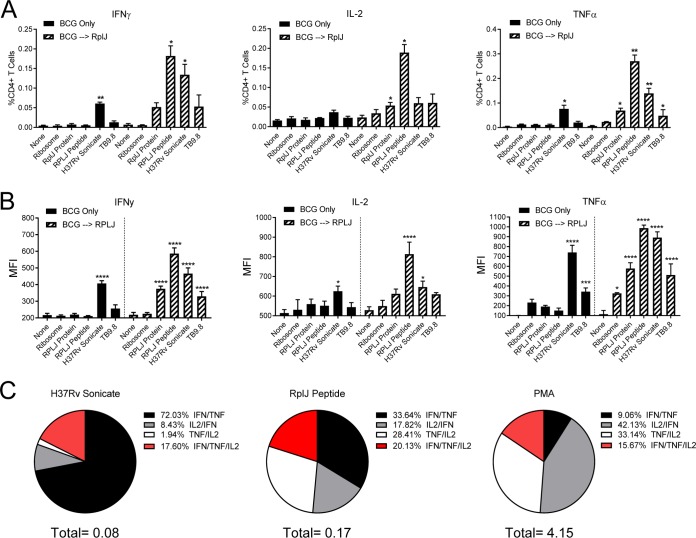
FIG 5.
Induction of Th1 multifunctional CD4+ T cells by boosting with RplJ protein. Mice (C57BL/6) (n = 4) were immunized with 5 × 106 CFU BCG s.c. or 5 × 106 CFU BCG s.c. followed 4 weeks later by two administrations of RplJ protein (25 μg) and CpG-ODN 1826 (20 μg) s.c. 2 weeks apart. Four weeks after the final immunization, single-cell suspensions of splenocytes were restimulated with recombinant RplJ protein (10 μg/ml), RplJTB146–160 peptide (10 μg/ml), TB9.8 peptide (10 μg/ml), H37Rv sonicate (10 μg/ml), or purified M. smegmatis ribosomes (5 nM). After 6 h of restimulation, cells were fixed and stained as described in Materials and Methods and analyzed by FACS analysis. Positive-control cells were stimulated with PMA plus ionomycin (10 μg/ml) (not shown). (A) Frequencies of CD4+ T cells producing each cytokine in immunized mice in response to antigen restimulation. (B) Mean fluorescence intensity (MFI) as an indication of the average level of cytokine production by CD4+ T cells in response to antigen restimulation. (C) Pie charts showing the relative fractions of dual- and triple-cytokine-producing CD4+ T cells following restimulation with the H37Rv sonicate, RplJ peptide, or PMA-ionomycin. The total below the pie charts is the fraction of total CD4+ T cells producing two cytokines. Percent values in the key represent the percentages of the total dual- and triple-cytokine-producing CD4+ T cells in the sample. Data shown represent mean values and standard errors for groups of four mice; responses that are significantly different from those with the no-antigen control by ANOVA are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).