Abstract
Fungal meningitis is most commonly causes by Cryptococcus species and dimorphic fungi. We present a rare case of mould meningitis, ventriculitis and subependymal nodules in an immunocompetent patient, having likely seeded the meninges and ventricular system through intravenous drug use. The causative mould remains undetermined. The case highlights the poor sensitivity of CSF culture and the need to consider surgical biopsy where there is diagnostic difficulty and fungal infection is being considered.
Keywords: Fungal meningitis, Intravenous drug use, IVDU, Pithomyces
1. Case
Cryptococcus neoformans and Cryptococcus gattii are the commonest organisms causing central nervous system (CNS) fungal infection, with major risks being HIV infection and corticosteroid use, though infection occurs in immunocompetent hosts. In contrast to other fungal CNS infections, a sensitive and specific test exists in the form of CSF cryptococcal antigen [1]. Treatment consists of at least 2 weeks of an amphotericin based product with concomitant flucytosine, with a consolidation phase of high dose fluconazole, followed by a maintenance phase of at least 6 months of lower dose fluconazole [2]. Several factors predict poor prognosis, such as the underlying disease, burden of yeasts as determined by the India ink smear or high antigen titres, raised intracranial pressure, and reduced level of consciousness at presentation [1]. Dimorphic fungi such as Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis are also common causes of CNS fungal infection occurring in particular geographic regions. Apart from the well documented outbreak of Exserohilum rostratum meningitis due to contamination of methylprednisolone used for spinal injections, meningitis due to moulds is rare [3]. From 18 September 2012 to 1 July 2013, 749 E. rostratum outbreak-associated cases and 6 deaths were reported to the Centers for Disease Control and Prevention. A selection of 328 cases investigated from the six most affected states of America showed a confirmatory laboratory diagnosis by culture, molecular assay or histopathology could be reached in only 36% of cases where samples were available. 81% had CNS infection, mostly meningitis, but there were also some patients with arachnoiditis or stroke (particularly involving the posterior circulation), while 19% had non-CNS infections such as epidural abscess, spinal osteomyelitis/discitis, facet joint infection or paraspinal infection. Cases were predominantly treated with liposomal amphotericin, voriconazole, or both agents together or sequentially. Death occurred in 26 of the 328 analysed cases, mostly due to stroke (22 cases) [3]. We present an isolated case of the rare entity of mould meningitis.
A 40 year old male was admitted on day 0 with headaches and confusion on a three month background of tonic clonic seizures, with a history of heavy alcohol and intravenous amphetamine use. On examination he was drowsy and confused but obeyed commands. He had no focal neurological deficits. Blood neutrophils were slightly elevated (8.1 × 109/L; N 2.0–7.5 × 109/L), C-reactive protein was 6.5 mg/L (N<5 mg/L). Serum sodium and calcium concentrations were normal. HIV serology was negative. Initial magnetic resonance imaging (MRI) showed an enlarged right lateral ventricle with enhancing cystic subependymal lesions and diffuse leptomeningeal enhancement of the basal cisterns and spinal cord (Fig. 1). CT of the chest, abdomen and pelvis revealed a renal mass that was subsequently confirmed by biopsy to be a clear cell carcinoma. There was no other evidence for metastatic disease.
Fig. 1.
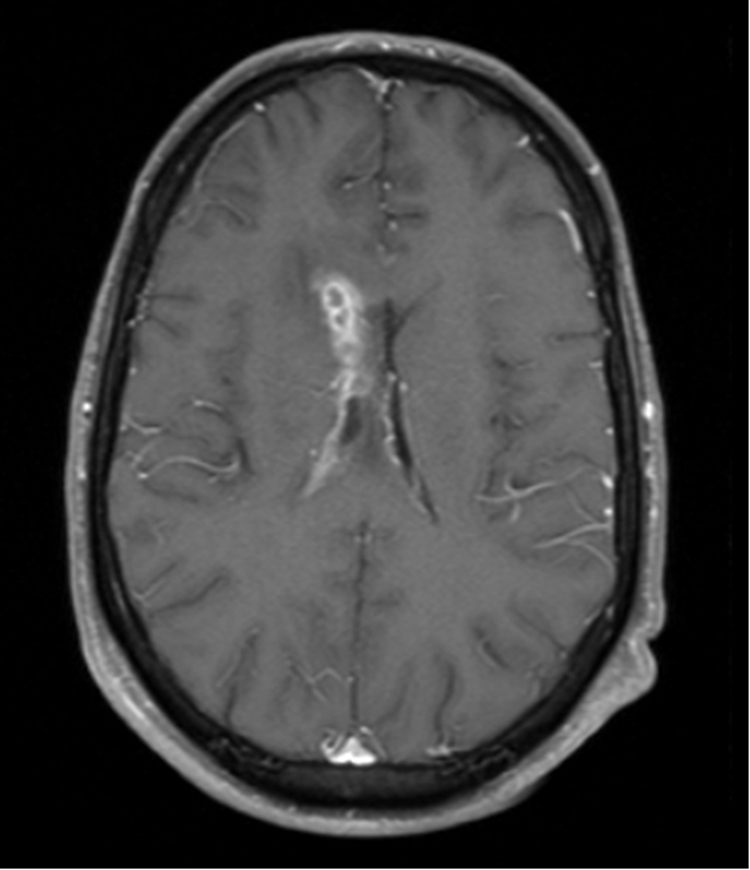
Initial MRI brain axial T1 image with gadolinium showing subependymal contrast-enhancing cystic lesions.
Examination of the cerebrospinal fluid (CSF) showed a white cell count of 18 × 106 cells/L (65% neutrophils, 35% mononuclear cells), erythrocyte count 52 × 106 cells/L, glucose 1.8 mmol/L, and protein 1.34 mg/L. Bacterial, fungal and mycobacterial microscopy and culture were negative. CSF cryptococcal antigen was negative. In-house CSF PCR assay results were negative for HSV, VZV, enterovirus species, Toxoplasma gondii, Mycobacterium tuberculosis complex, bacterial 16S rRNA sequencing and fungal internal transcribed region (ITS) sequencing. Taenia solium (serum and CSF), Trichenella spiralis (serum) and Echinococcus spp (serum) IgG were negative. Hydatid hooklets were not seen in CSF. CSF cytology showed a mixture of histiocytes, neutrophils and mononuclear cells. No abnormal lymphocyte population was detected by flow cytometry of the CSF.
Following admission he became progressively drowsy and repeat imaging confirmed progressive hydrocephalus which was initially treated with external ventricular drainage. It soon became apparent that he was drain dependent and subsequently had bilateral ventriculo-peritoneal shunts inserted day+11.
Differential diagnoses included leptomeningeal carcinomatosis related to the renal cell cancer, neurosarcoidosis (serum angiotensin converting enzyme {ACE} 22 U/L; N 20–70 U/L, CSF ACE 12.2 U/L; N<4 U/L, not hypercalcemic, no chest infiltrates or lymphadenopathy), tuberculous or fungal meningitis knowing the imperfect sensitivity of CSF culture and PCR, or an unusual organism seeding the meninges via intravenous drug use.
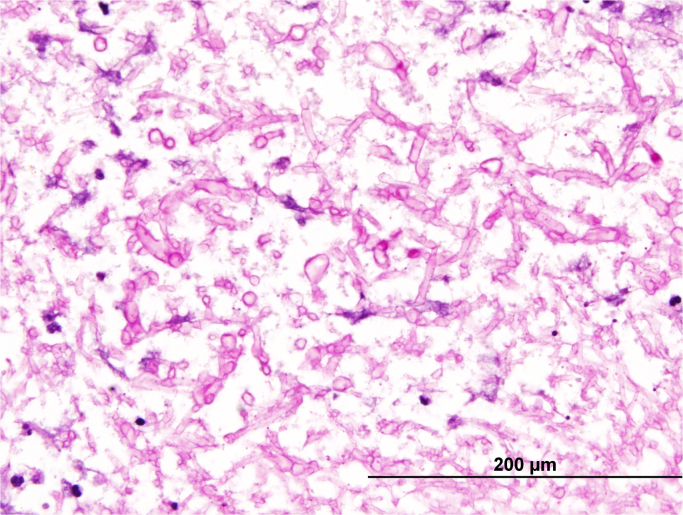
The cause for meningitis, ventriculitis and subependymal nodules remained undetermined and there was clinical deterioration with progressive enlargement of the subependymal lesions, such that at day+90 he proceeded to open surgical brain biopsy of the enhancing right lateral ventricle and overlying dura. Histopathological examination of the brain revealed suppurative necrotic material with septate non-pigmented hyphal elements (Fig. 2.). The dura showed fibrosis with reactive change and some epithelioid histiocytes however no fungal organisms were seen.
Fig. 2.
Brain biopsy showing numerous septate hyphal elements (Periodic acid-Schiff stain, x60).
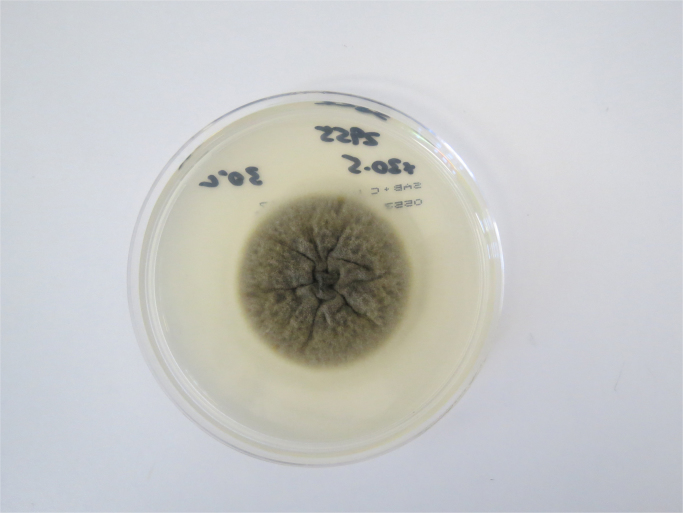
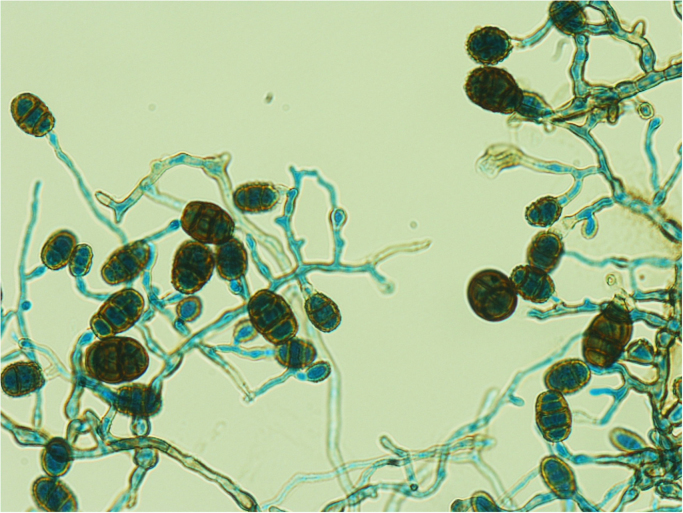
Limited sample was received in microbiology, which was cultured on blood agar and chocolate agar at 35 °C in CO2, blood agar at 35 °C anaerobically, 7H10 at 35 °C in air, Sabouraud Dextrose agar with chloramphenicol (SAB-C) at 30 °C and 26 °C in air. Despite extended incubation of all bacterial and fungal media, only a single fungal colony was isolated at 10 days on the chocolate agar which had been transferred to 30 °C for the extended incubation period. Morphological studies of the isolate on Potato Dextrose Agar demonstrated a dematiaceous hyphomycete (Fig. 3.), with hyphae variable from hyaline tending to become dematiaceous, becoming more densely septate and echinulate. Spores were echinulate, obovoid, becoming distinctly pigmented with transverse and longitudinal septae (Fig. 4.). ITS sequencing of the fungus by an in-house assay showed 100% homology with Pithomyces chartarum (GenBank accession number MF374508), being consistent with the morphological features described. Direct fungal (ITS) sequencing from the brain sample did not yield a molecular product. Filamentous fungal (mould) meningitis and ventriculitis with subependymal involvement was the confirmed diagnosis from the histopathological findings, however as the growth was only on a single plate and the fungus did not grow at body temperature (37 °C), it is unclear whether Pithomyces chartarum was the causative mould.
Fig. 3.
Macroscopic appearance of Pithomyces chartarum on Sabouraud Dextrose Agar with chloramphenicol after 8 days at 30 °C.
Fig. 4.
Microscopic appearance of Pithomyces chartarum from culture showing hyaline to dematiaceous echinulate hyphae with spores which are pigmented echinulate, obovoid, containing transverse and longitudinal septae (Lactophenol Cotton Blue stain, x400).
Oral voriconazole was commenced day+98 with two 400 mg loading doses on the first day, then 200 mg bd (patient weight 59 kg). Trough voriconazole level was 1.5 mg/L day+102, and the patient was lost to follow up for a period but claimed to be compliant with voriconazole 200 mg bd. When next measured at day+270, the voriconazole trough level was low at 0.3 mg/L. The dose was increased to 300 mg bd, and with a subsequent level of 5.3 mg/L at day+300 he continued on 300 mg bd. He had clinically and radiologically improved at the most recent review at day+630 when we decided to continue voriconazole but we have not been able to obtain another level. Formal neurocognitive assessment has not been performed however he reported full recovery aside from minor memory impairment and occasional unsteadiness of gait. Most recent MRI at day+630 showed resolution of the subependymal enhancement and cystic lesions and normal ventricular size. Bilateral ventriculo-peritoneal shunts remain in situ.
2. Discussion
Fungal meningitis usually occurs from haematogenous spread (e.g. from a pulmonary focus or cardiac valve), direct extension from a contiguous focus such as the sinuses, or direct inoculation from trauma or contaminated spinal injection 1, 4. In our case, none of these secondary sites were found. Given that he was immunocompetent, we postulate that the patient had injected fungus through illicit drug use which subsequently seeded the meninges, a mechanism previously described for meningitis and ventriculitis due to Candida, Aspergillus, Mucorales, Cryptococcus among other fungi 5, 6, 7, 8.
This case illustrates the difficulty establishing a microbiological diagnosis, a consequence of the low concentration of organism that can be present in the CSF (e.g. <1 colony forming units/mL) requiring large volumes for culture diagnosis-10–30 mL has been suggested [1]. Despite obtaining large volumes of CSF on several occasions, cultures were negative, and we could not definitively identify the causative mould by culture and PCR of brain tissue, however the small amount of brain tissue sent for these microbiological tests was likely a contributing factor.
Because Pithomyces chartarum was only grown from one plate and not detected by direct molecular testing, it is not clear if it was definitely the causative fungus. This dematiaceous mould has been reported to cause facial eczema in animals, and there are reports of onychomycosis and peritonitis in humans. The largest report of 42 Pithomyces human, animal and environmental specimens included some human skin, nail, and respiratory tract samples presumably representing colonisation. Low mean inhibitory concentrations for amphotericin, triazoles and echinocandins were demonstrated [9].
We chose voriconazole to treat this patient as it has a high level of central nervous system penetration and an acceptable side effect profile. It is suggested first line treatment for CNS aspergillosis [10], has in vitro activity against Pithomyces species, and a broad range of other moulds which may cause fungal meningitis (e.g. Scedosporium apiospermum, Fusarium species, some dematiaceous moulds). It lacks activity against Mucorales, however the histological features were not suggestive of infection with this fungal group, which is characterised by aseptate, broad ribbon-like hyphal elements. Voriconazole is the mould active antifungal with the highest CSF penetration, a consequence of lower molecular size and protein binding relative to other antifungals. However it should be noted there is poor correlation between antifungal concentrations in CSF compared to brain parenchyma, and also a lack of data correlating CSF antifungal concentrations with drug efficacy [11]. The required duration of antifungal treatment is uncertain in this case but should be prolonged-no duration is offered in guidelines of CNS aspergillus infections [10] and at least 9 months is recommended for cryptococcal meningitis [2].
Mortality with mould meningitis is generally >50% [1]. A 52% mortality was reported for Aspergillus meningitis [12], in part a reflection of a cohort of patients including disseminated mould infection and a high percentage who were immunocompromised. The rate of neurological recovery has not been documented.
Here we present a case of mould meningitis in an immunocompetent patient, having likely seeded the meninges and ventricular system through intravenous drug use. The case highlights the poor sensitivity of CSF culture and the need to consider surgical biopsy where there is diagnostic difficulty and fungal infection is being considered. It also highlights the need for prolonged antifungal therapy and the possibility of neurological recovery.
Acknowledgements
Thanks to Matthew Skinner and Lay Kho who assisted with the diagnosis and management of the patient.
Acknowledgments
Conflicts of interest and sources of funding
The authors state that there are no conflicts of interest to disclose.
References
- 1.Gottfredsson M., Perfect J.R. Fungal meningitis. Semin. Neurol. 2000;20(3):307–322. doi: 10.1055/s-2000-9394. [DOI] [PubMed] [Google Scholar]
- 2.Perfect J.R., Dismukes W.E., Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiller T.M., Roy M., Nguyen D. Clinical findings for fungal infections caused by methylprednisolone injections. N. Engl. J. Med. 2013;369:1610–1619. doi: 10.1056/NEJMoa1304879. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti A. Epidemiology of central nervous system mycoses. Neurol. India. 2007;55(3):191–197. doi: 10.4103/0028-3886.35679. [DOI] [PubMed] [Google Scholar]
- 5.Morrow R., Wong B., Finkelstein W.E. Aspergillosis of the cerebral ventricles in a heroin abuser: case report and review of the literature. Arch. Intern. Med. 1983;143:16. [PubMed] [Google Scholar]
- 6.Kaufman D.M., Thal L.J., Farmer P.M. Central nervous system aspergillosis in two young adults. Neurology. 1976;26:484. doi: 10.1212/wnl.26.5.484. [DOI] [PubMed] [Google Scholar]
- 7.Monno L., Angarano G., Montagna M.T. Chronic cryptococcal meningitis in an intravenous drug addict without evidence of infection by HIV-1,2 in Southern Italy. Eur. J. Epidemiol. 1994;10(6):773–774. doi: 10.1007/BF01719297. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins R.J., Rothman M., Fiore A. Central mucormycosis associated with intravenous drug use: three case reports and review. Clin. Infect. Dis. 1994;19:1133–1137. doi: 10.1093/clinids/19.6.1133. [DOI] [PubMed] [Google Scholar]
- 9.da Cunha K.C., Sutton D.A., Gene J. Pithomyces species (Montagnulaceae) from clinical specimens: identification and antifungal susceptibility profiles. Med. Mycol. 2014;52:748–757. doi: 10.1093/mmy/myu044. [DOI] [PubMed] [Google Scholar]
- 10.Patterson T.F., Thompson G.R., Denning D.W. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kethireddy S., Andes D. CNS pharmokinetics of antifungal agents. Exp. Opin. Drug Metab. Toxicol. 2007;3(4):573–581. doi: 10.1517/17425225.3.4.573. [DOI] [PubMed] [Google Scholar]
- 12.Kourkoumpetis T.K., Desalermos A., Muhammed M. Central nervous system aspergillosis. Medicine (Baltim.) 2012;6:328–336. doi: 10.1097/MD.0b013e318274cd77. [DOI] [PubMed] [Google Scholar]