Abstract
Scleroderma is a multisystem fibroproliferative disease with no effective medical treatment. Myofibroblasts are critical to the fibrogenic tissue repair process in the skin and many internal organs. Emerging data support a role for both matrix stiffness, and transforming growth factor β1 (TGFβ1), in myofibroblast differentiation. Transient receptor potential vanilloid 4 (TRPV4) is a mechanosensitive ion channel activated by both mechanical and biochemical stimuli. The objective of this study was to determine the role of TRPV4 in TGFβ1- and matrix stiffness-induced differentiation of dermal fibroblasts. We found that TRPV4 channels are expressed and functional in both human (HDF) and mouse (MDF) dermal fibroblasts. TRPV4 activity (agonist-induced Ca2+ influx) was induced by both matrix stiffness and TGFβ1 in dermal fibroblasts. TGFβ1 induced expression of TRPV4 proteins in a dose-dependent manner. Genetic ablation or pharmacological antagonism of TRPV4 channel abrogated Ca2+ influx and both TGFβ1-induced and matrix stiffness-induced myofibroblast differentiation as assessed by 1) α-smooth muscle actin expression/incorporation into stress fibers, 2) generation of polymerized actin, and 3) expression of collagen-1. We found that TRPV4 inhibition abrogated TGFβ1-induced activation of AKT but not of Smad2/3, suggesting that the mechanism by which profibrotic TGFβ1 signaling in dermal fibroblasts is modified by TRPV4 may be through non-Smad pathways. Altogether, these data identify a novel reciprocal functional link between TRPV4 activation and TGFβ1 signals regulating dermal myofibroblast differentiation. These findings suggest that therapeutic inhibition of TRPV4 activity may provide a targeted approach to the treatment of scleroderma.
Keywords: TRPV4, calcium influx, dermal myofibroblast differentiation, matrix stiffness
fibrosis, a form of scarring, is a common pathological process in the lung, heart, kidney, vasculature, skin, and liver (5, 15, 36, 40, 47, 48). Fibrosis is responsible for nearly half of deaths in the developed world, yet, there is no effective anti-fibrotic therapy (54). Fibroblasts-to-myofibroblasts transition is a key event in maintaining tissue homeostasis and in both physiological and pathological tissue repair processes. In fibrotic and scarring pathologies like scleroderma, sustained and exacerbated differentiation of fibroblasts into myofibroblasts is an essential component of the synthesis and deposition of extracellular matrix (ECM) responsible for excessive scarring or fibrosis (5, 15, 47, 48). Fibroblasts directly respond to changes to their surrounding mechanical environment during myofibroblast differentiation (48, 51). For example, in response to acute or chronic lung injury, myofibroblasts accumulate in high matrix stiffness areas known as fibroblastic foci (13, 47, 52). The contribution of mechanical stress in augmenting dermal myofibroblast activity has also been shown experimentally by stretching dermal wounds in mice, which results in increased scar formation (38, 52). Although both mechanical and biochemical signals are essential for optimal fibroblast differentiation, the precise mechanisms and the identity of plasma membrane mechanosensors by which differentiation signals are transduced and propagated are unknown. Compelling evidence indicates that cell adhesion, integrin, and focal adhesion kinase (FAK) signaling are critical for fibroblast differentiation in vitro and in vivo (14, 25, 38, 48, 51–53). Although FAK has been shown to transmit mechanotransduction signals that induce dermal fibroblast differentiation (53), the identity of a mechanotransduction receptor/channel upstream of FAK activation, and the molecular mechanisms by which mechanical signals are transduced intracellularly to drive fibroblast differentiation, remain unknown. Recently, we found that TRPV4, a mechanosensitive, Ca2+-permeable, plasma membrane channel, mediates myofibroblast differentiation of lung fibroblasts and contributes to the development of pulmonary fibrosis in a mouse model (39). TRPV4 has not previously been known to be involved in dermal myofibroblast differentiation, nor has the mechanism of its involvement in this process been determined.
TGFβ1 is the most well-studied profibrotic mediator of both myofibroblast differentiation in vitro, and of fibrogenesis of many organs including skin (2, 4, 5, 40, 47, 55). TGFβ1 signals through a plasma membrane receptor complex of TGFβ1-receptor type I and II resulting in downstream activation of either the transcription factors Smad2/3 pathways or through Smad-independent pathways that include c-Jun NH2-terminal kinase, p38 kinases, FAK, phosphatidylinositol 3-kinase (PI3K), and Rho GTPases. Both Smad and non-Smad pathways have been linked to myofibroblast differentiation and fibrosis (2, 4, 55). TGFβ1 also has beneficial/homeostatic roles in regulating immune suppression, inflammation, cell growth, and tissue maintenance in various pathophysiological conditions (2, 4, 55). Therefore, any therapeutic approach that directly targets TGFβ1 is likely to have serious, perhaps fatal, side effects. However, a specific molecular strategy that targeted the fibrogenic signals of the TGFβ1 pathway without interfering with the beneficial roles of TGFβ1 would have considerable therapeutic potential. The potential cross-talk of matrix stiffness and soluble TGFβ1 in TRPV4 signaling in dermal fibroblasts may provide such an opportunity.
TRPV4, a member of the TRP superfamily, is ubiquitously expressed in various cell types including fibroblasts. TRPV4 is activated by a wide range of chemical and physical stimuli including changes in osmolarity, warm temperatures, mechanical stresses, UVB exposure, growth factors, and metabolites of arachidonic acid (12, 22, 24, 32, 37, 39, 50, 57). Intriguingly, TRPV4 channels has been shown to be activated by cyclical stretching of cells, or by mechanical forces applied to β1 integrins in cell surface (27, 45). Recently, TRPV4 was shown to function as a mechanosensor in response to dynamic loading in chondrocytes (37). In addition to its transmembrane structure, TRPV4 contains a number of potential regulatory domains and protein-interaction sites, including PI3K and Src homology 2 (SH2) recognition domains, putative protein kinase C (PKC) phosphorylation sites, putative PDZ domains, and an NH2-terminal ankyrin repeat region (9, 13). Various gain- and loss-of-function mutations in TRPV4 have been linked to human diseases including skeletal dysplasia and sensory and motor neuropathies (3, 8, 18). TRPV4 is linked to multiple physiological and pathological functions including sheer stress detection in blood vessels, joint diseases, skeletal malformations, pain response, and osteoclast differentiation control in bone (7, 16, 17, 19, 26, 28). In mice, TRPV4 deficiency is associated with altered blood pressure and vasodilatory responses, vascular permeability, bladder function, lung injury, and lung fibrosis (3, 8–10, 13, 18, 29, 39). However, a potential role of TRPV4 mechanosensing in dermal myofibroblast differentiation has not been reported. In this study we sought to determine the expression and mechanosensing role of TRPV4 calcium channels in dermal myofibroblast differentiation. Results presented here identify a novel regulatory role for TRPV4 in dermal myofibroblast differentiation induced by both TGFβ1 and pathophysiological matrix stiffness.
MATERIALS AND METHODS
Animal and cell culture.
TRPV4 knockout (TRPV4 KO) mice on a C57BL/6 background were originally generated by Dr. M. Suzuki, Department of Pharmacology, Jichi Medical University, Tochigi, Japan (31). We acquired TRPV4 KO mice from Dr. D. X. Zhang, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI (56). Congenic wild-type (WT) C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). All experiments on mice were performed in accordance with the Institutional Animal Care and Use Committee guidelines and guidelines approved by the University of Maryland review committee. Mice were housed under pathogen-free conditions with controlled temperature and humidity, and with food and water available ad libitum.
Primary normal adult human dermal fibroblasts (HDFs) were purchased from ATCC (Manassas, VA), and were used within passages 3–8 for all experiments. Primary normal mouse dermal fibroblasts (MDFs) were derived from skin biopsies from armpit of 8- to 10-wk-old WT and TRPV4 KO mice as described previously (21). Both HDFs and MDFs were maintained in Minimum Essential Media (MEM) supplemented with 10% fetal bovine serum (FBS), and penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA). Cells were incubated at 37°C/5% CO2 and subcultured at 70% confluence. For isolation of MDFs, mouse skin tissue was washed with HBSS (with Ca2+), minced, and treated with collagenase (2.5 mg/ml) for 30 min with continuous shaking at 37°C/5% CO2. Digested tissues were spun down, resuspended in MEM 15% FBS, and mechanically dissociated by pipetting up and down 8–10 times. The fibroblasts isolated from the tissue were then seeded in culture flasks. Medium was replaced with MEM 10% FBS every 48 h thereafter. Although the cultures initially contained a variety of cell types including macrophages, keratinocytes, and adipocytes, the procedure and culture conditions selectively promote fibroblast growth. Repeated trypsinization and culturing of cells in MEM removes keratinocytes. The polyhedral and cuboidal shaped keratinocytes can be easily distinguished from elongated and spindle-shaped fibroblasts. We checked for the presence of fibroblast markers such as α-smooth muscle actin (α-SMA) and incorporation of α-SMA into F-actin stress fibers by immune/phalloidin double staining. For all experiments, HDFs and MDFs were seeded on collagen-coated (10 µg/ml) surfaces in MEM 1% FBS for 24 h followed by treatment with TGFβ1 (R&D Systems, Minneapolis, MN) in serum-free medium containing 1% bovine serum albumin (BSA).
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was extracted from HDFs and MDFs using RNeasy micro kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RT-PCR was carried out under a standard protocol using the following TRPV4 primers: forward, 5′-GGCACCTATCGTCACCACTC-3′, and reverse, 5′-TCCTCATCAGTTAGGCGTTTC-3′; and mouse TRPV4 primers: forward, 5′-GAGTCCTCAGTAGTGCCTGG-3′, and reverse, 5′-CAACAAGAAGGAGAGCAGTC-3′.
Western blot analysis.
After treatment with TGFβ1, with or without inhibitors (TRPV4 inhibitor: GSK219; and PI3K inhibitors: LY294002 and wortmannin), cells were lysed in modified RIPA buffer (50 mM Tris·HCl, 150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) with protease and phosphatase inhibitors. Cell lysates were resolved on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Immunoblotting was performed using the following primary antibodies: rabbit anti-phospho-Smad2/3, rabbit anti-Smad2/3, rabbit anti-phospho-Akt, rabbit anti-Akt (1:2,000; Cell Signaling, Beverly, MA); mouse anti-α-SMA (1:20,000; Sigma, St. Louis, MO); goat anti-collagen type I (1:700; EMD Millipore, Billerica, MA); rabbit anti-TRPV4 (1:700; Alomone, Jerusalem, Israel), goat anti-β-actin (EMD Millipore); rabbit anti-GAPDH (1:3,000; Santa Cruz, Dallas, TX), followed by incubation with secondary antibodies goat anti-mouse, goat anti-rabbit, and donkey anti-goat (1:5,000; Jackson ImmunoResearch, West Grove, PA). Immunoreactive bands were visualized using an enhanced chemiluminescence system (UVP Biospectrum, Upland, CA).
In vitro myofibroblast differentiation assay.
To assess myofibroblast differentiation in MDFs and HDFs, cells were seeded on hydrogels (0.5 and 8 kPa) (Matrigen Life Technologies, Brea, CA) or coverglasses. Cells were maintained in MEM 1% FBS for 24 h. Cells were then incubated in serum-free MEM supplemented with 1% BSA with or without TGFβ1 (2 or 5 ng/ml) and TRPV4 antagonists GSK2193874 (GSK219), and HC-067047 (HC) (Sigma) or vehicle, for 48 h and then processed for immunofluorescence staining of myofibroblast markers.
Immunofluorescence staining analysis.
Immunofluorescence staining was performed for colocalization of the structural elements of dermal fibroblasts as previously described (55). Cells adhered on coverglasses or hydrogels were fixed with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100 and blocked with 3% BSA/PBS. To examine α-SMA incorporation into cytoskeletal fibers or TRPV4 protein expression, samples were incubated with anti-α-SMA (1:400) or anti-TRPV4 (1:50), followed by incubation with goat anti-mouse Alexa Fluor 488 (1:300; Thermo Fisher Scientific). F-actin fibers were stained using Alexa Fluor 594 phalloidin (1:300; Thermo Fisher Scientific). Cells were then mounted using Prolong diamond antifade reagent with DAPI (Thermo Fisher Scientific). Immunofluorescence intensity was quantified using ImageJ software (NIH), and the results are presented as Integrated Density or Int Density (the product of area and mean gray value).
Measurement of intracellular calcium.
Changes in intracellular calcium in dermal fibroblasts were detected by the FlexStation system using a FLIPR calcium 5 Assay Kit (Molecular Devices, Sunnyvale, CA). Briefly, HDFs or MDFs (10,000 cells/well in 1% MEM) were treated for 48 h with or without TGFβ1 at 37°C, 5% CO2. Cells were then incubated with FLIPR kit reagents (Calcium 5 dye in 1X HBSS solution containing 20 mM HEPES and 2.5 mM probenecid) for 45 min at 37°C. Cells were incubated with vehicle or TRPV4 antagonist, GSK219, for 45 min at 37°C and then the 96-well plate was transferred to the FlexStation for measuring intracellular calcium change (Ca2+ influx). Calcium influx was induced by the TRPV4 agonist, GSK1016790A (GSK101), in fibroblasts that were pretreated with vehicle or GSK219, and recorded by measuring ΔF/F (Max-Min); data are shown as relative fluorescence units (RFU). The effect of matrix stiffness on Ca2+ influx in MDFs and HDFs was assayed using hydrogels of different stiffness (1, 8, and 25 kPa) bound to 96-well glass bottom plates (39). Measurement of Ca2+ influx was performed using the FlexStation system.
Statistics.
All data are expressed as means ± SE as indicated below. Statistical comparisons between control and experimental groups were performed with the Student’s t-test or one-way ANOVA using SigmaPlot or Prism software; P < 0.05 was considered significant.
RESULTS
TRPV4 protein is expressed and functional channels occur in both primary human and murine dermal fibroblasts.
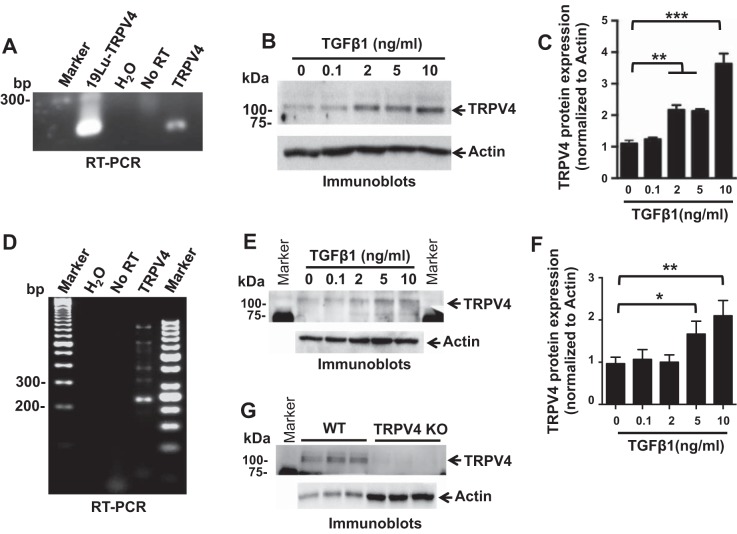
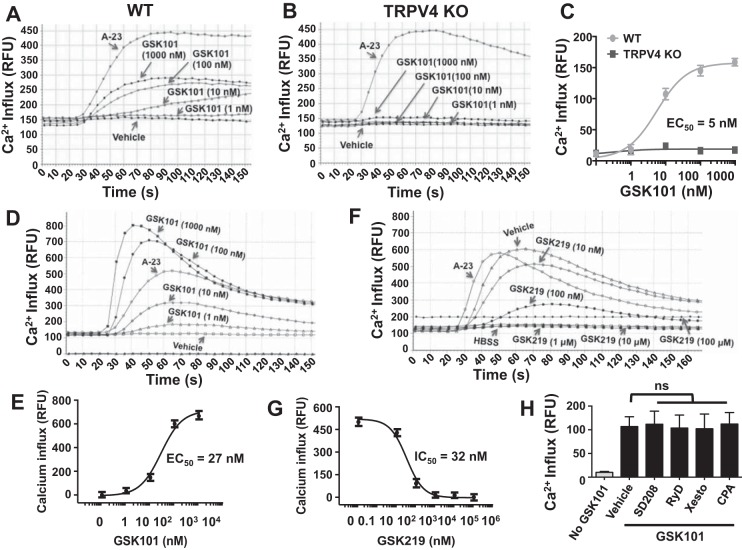
To determine whether TRPV4 channels are required in dermal myofibroblast differentiation, we first examined TRPV4 expression in primary normal human and mouse dermal fibroblasts (HDFs and MDFs). We detected expression of TRPV4 RNA in HDFs using reverse transcription polymerase chain reaction (RT-PCR) analysis (Fig. 1A), and we detected expression of TRPV4 protein in HDFs in the presence or absence of TGFβ1 by immunoblotting (Fig. 1B) (7, 23). Stimulation of HDFs with TGFβ1 for 48 h produced a dose-dependent increase in total cellular TRPV4 protein expression (Fig. 1C). Similarly, we detected expression of TRPV4 RNA in MDFs using RT-PCR analysis (Fig. 1D), and we detected expression of TRPV4 protein in MDFs in response to TGFβ1 doses by immunoblotting (Fig. 1E). Stimulation of MDFs with TGFβ1 for 48 h produced a dose-dependent increase in total cellular TRPV4 protein expression (Fig. 1F). We validated the TRPV4 antibody by immunoblot analysis showing TRPV4 protein expression in wild-type (WT) MDFs, which is absent in TRPV4 KO MDFs (Fig. 1G). To confirm the presence of functional Ca2+-permeable TRPV4 channel in dermal fibroblasts, we compared Ca2+ influx in primary mouse dermal fibroblasts (MDFs) from TRPV4 knockout (TRPV4 KO) and congenic wild-type (WT) mice over a range of concentrations of a selective TRPV4 agonist, GSK101 (1–1,000 nM) (10). GSK101-induced Ca2+ influx was undetectable in TRPV4 KO fibroblasts even in the presence of a 200-fold excess of GSK101 (EC50 = 5 nM) (Fig. 2, A–C), suggesting the absence of redundant GSK101-responsive Ca2+ influx channels in MDFs. To test whether TRPV4 channel activation induces Ca2+ influx (cytosolic calcium increases) in HDFs, we measured Ca2+ influx induced by a TRPV4 agonist. We detected a rapid (within 40–70 s), concentration-dependent increase in intracellular Ca2+ influx in HDFs in response to GSK101 (EC50 = 27 nM) (Fig. 2, D and E). GSK101-induced Ca2+ influx was inhibited in a concentration-dependent manner (IC50 = 32 nM) by pretreatment with the selective small molecule TRPV4 antagonist, GSK219 (46) (Fig. 2, F and G). As expected from work in human lung fibroblasts (HLFs) (39), the TRPV4-mediated rise in intracellular Ca2+ was entirely dependent on the presence of external Ca2+ in HDFs (Fig. 2H). TGFβ1 stimulation can cause calcium wave activity in HLFs that is dependent on both an external influx of Ca2+ and on the release of Ca2+ from internal stores (34). However, we found that TRPV4-dependent Ca2+ influx in HDFs was not blocked by pretreatment of cells with specific small molecule inhibitors of TGFβRI, inhibitors of inositol 1,4,5-trisphosphate receptor (IP3R), inhibitors of ryanodine receptors (RyR), or inhibitors of the sarco/endoplasmic reticulum calcium transport ATPase (SERCA) (SD208 for TGFβRI, Xesto for IP3R, RyD for RyR, and CPA for SERCA) (Fig. 2H). These results suggest that the mobilization of internally released Ca2+ is not necessary for TRPV4-elicited Ca2+ influx in HDFs. These results indicate that both human and murine dermal fibroblasts express functional TRPV4 channels capable of mediating calcium influx resulting in a rise in intracellular calcium.
Fig. 1.
TRPV4 is expressed in primary normal human (HDFs) and mouse (MDFs) dermal fibroblasts, and stimulation of HDFs or MDFs with TGFβ1 produced a dose-dependent increase in total cellular TRPV4 protein expression. A: RT-PCR analysis demonstrating that TRPV4 mRNAs are present in HDFs. H2O (no RNA) and No RT (no reverse transcriptase) samples were used as negative control, whereas RNA extracted from human lung fibroblast cells (19Lu) was used as positive control. B: representative immunoblots show TRPV4 protein expression in HDFs treated with indicated doses of TGFβ1 for 48 h. Total actin was used as loading control. C: quantitative analysis of total cellular TRPV4 protein expression in TGFβ1-treated HDFs. Results shown are means ± SE from 3 independent experiments (**P < 0.01, ***P < 0.001 for TGFβ1-treated cells vs. untreated, n = 3, t-test). D: RT-PCR analysis demonstrating that TRPV4 mRNAs are present in MDFs. H2O (no RNA) and No RT (no reverse transcriptase) samples were used as negative control. E: representative immunoblots show TRPV4 protein expression in MDFs treated with indicated doses of TGFβ1 for 48 h. Total actin was used as loading control. F: quantitation of results from E. Results shown are means ± SE from 3 independent experiments (*P < 0.05, **P < 0.01 for TGFβ1-treated cells vs. untreated, n = 3, t-test). G: immunoblots show TRPV4 protein expression in wild-type (WT) MDFs, which is absent in TRPV4 KO MDFs. Triplicate samples were loaded for analysis and actin was used as loading control. For experiments described in this figure, cells were seeded on collagen-coated (10 μg/ml) plastic plates.
Fig. 2.
TRPV4 calcium channel is functional in MDFs and in HDF cells. A and B: FlexStation 3 recording of Calcium 5 dye-loaded WT MDF monolayers shows that TRPV4 agonist GSK101 induces Ca2+ influx in a concentration-dependent manner, which is completely absent in TRPV4 KO MDFs. C: quantitation of results (means ± SE) from A and B. All experiments were repeated 3 times in quadruplicate. D: HDFs (10,000 cells per well) were seeded on collagen-coated (10 μg/ml) 96-well plastic plate. Ca2+ influx is shown by relative fluorescence units (RFUs) measuring ΔF/F (Max-Min). A23 (2 μM), a calcium ionophore, was used as a positive control. FlexStation 3 recording of Calcium 5 dye-loaded HDF monolayers shows that TRPV4 agonist GSK101 induces Ca2+ influx in a concentration-dependent manner. E: quantitation of results from D (means ± SE). F: HDFs (10,000 cells per well) were seeded on collagen-coated (10 μg/ml) 96-well plastic plate. Concentration-dependent inhibition of TRPV4-elicited Ca2+ influx in HDFs by its selective antagonist, GSK219. TRPV4-elicited Ca2+ influx was generated by GSK101 (10 nM). All experiments were repeated 3 times in quadruplicate. G: quantitation of results from F (means ± SE). H: TRPV4-elicited calcium rise is not dependent on intracellular pools and/or regulators of calcium. HDF monolayers were compared for their intracellular calcium rise after TRPV4 activation by its specific agonist, GSK101 (10 nM), with or without selective inhibitors to TGFβRI (SD208, 10 μM), the inositol 1,4,5-triphosphate receptor [xestospongin C (Xesto), 10 μM], ryanodine receptors [ryanodine (RyD), 10 μM], or sarco/endoplasmic reticulum calcium transport ATPase [cyclopiazonic acid (CPA), 10 μM]. Results are expressed as means ± SE.
TGFβ1 stimulation and increased matrix stiffness potentiates TRPV4-mediated Ca2+ influx in dermal fibroblasts.
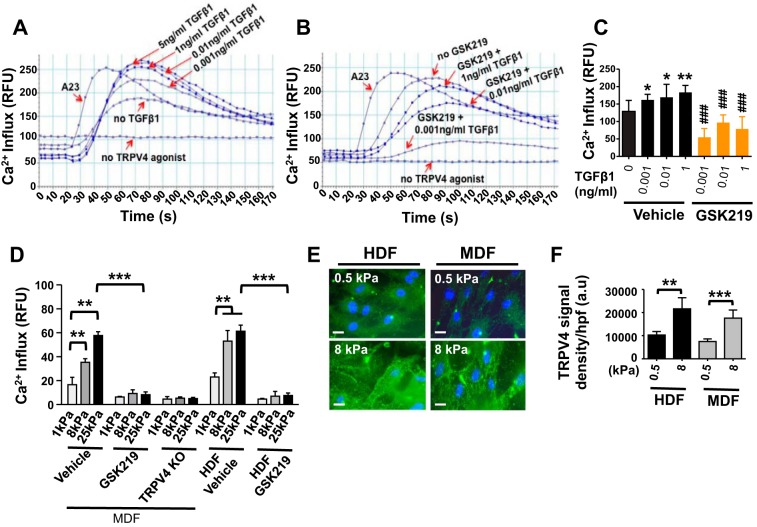
Emerging data support a role for both a mechanical signal, e.g., matrix stiffness, and a biochemical signal, e.g., TGFβ1, in fibroblast differentiation during the fibrotic process in multiple organs (14, 37, 48, 51, 52). However, the mechanism by which the matrix stiffness signal is transduced intracellularly and the identities of plasma membrane mechanosensors by which myofibroblast differentiation signals are propagated are unknown. Recently, we discovered that TRPV4 is required for both TGFβ1- and matrix stiffness-induced lung fibroblast differentiation and pulmonary fibrosis development in mice (39). Herein, we observed that pretreatment of HDFs with TGFβ1 caused a ~2-fold increase in GSK101-induced Ca2+ influx compared with untreated controls, and this response was inhibited by the TRPV4 antagonist, GSK219 (Fig. 3, A–C). In addition, we found that TRPV4 channel activity (as measured by agonist-induced Ca2+ influx) was increased in a matrix stiffness-dependent manner when cells were plated on stiffer matrices within the pathophysiological range (1–25 kPa) (Fig. 3D). Antagonism of the TRPV4 channel by GSK219, or genetic deletion of TRPV4 in MDFs, selectively abrogated matrix stiffness-induced Ca2+ influx (Fig. 3D). Since internal stiffness of cells is known to be modulated by stiffness of the matrix (43), it is possible that fibroblasts seeded on hydrogels (1, 8, or 25 kPa) generate reduced cellular stiffness. In contrast, cells may experience increased stiffness when seeded on plastic (stiffness > 1 GPa). As TRPV4 is a matrix stiffness-sensitive channel, the increased Ca2+ influx noted in fibroblasts seeded on plastic compared with 1, 8, or 25 kPa stiffness gels is possibly due to differences in differential activation of TRPV4 channels. A recent report from our group showed that Ca2+ influx in human lung fibroblasts was comparatively lower (<60) on 1, 8, or 25 kPa gel than on plastic (39), which supports our data here. Together, these results indicate that TRPV4 activity (Ca2+ influx) is potentiated by both TGFβ1 stimulation and matrix stiffness in dermal fibroblasts. To understand the mechanism whereby increased matrix stiffness can augment TRPV4 activity in dermal fibroblasts we determined if TRPV4 abundance in dermal fibroblasts increased in response to increasing matrix stiffness. For this, both HDFs and MDFs were plated on collagen-coated (10 μg/ml) hydrogels with varying degrees of stiffness (0.5 and 8 kPa). We found that TRPV4 protein expression is increased by matrix stiffness (8 kPa vs. 0.5 kPa) in both HDFs and MDFs (Fig. 3, E and F).
Fig. 3.
TGFβ1 and increased matrix stiffness augments TRPV4-dependent Ca2+ influx and its expression in dermal fibroblasts. A and B: GSK101-induced (10 nM) Ca2+ influx, measured as in Fig. 2, is augmented by pretreatment (48 h) of HDFs with increasing doses of TGFβ1 (as indicated) and abrogated by GSK219. A23 and untreated HDFs were used as positive and negative controls, respectively. C: the quantified results shown are means ± SE from 3 independent experiments (*P < 0.05, **P < 0.01 for TGFβ1 cells vs. untreated, and ###P < 0.001 for GSK219-treated cells vs. untreated). All experiments were repeated 3 times in quadruplicate. D: WT MDFs, TRPV4 KO MDFs, and HDFs were plated (10,000 cells per well) on collagen-coated (10 μg/ml) hydrogels with varying degrees of stiffness (1, 8, and 25 kPa) under vehicle-treated or GSK219-treated (5 μM) conditions. GSK101-induced (10 nM) Ca2+ influx, measured as in Fig. 2, is increased by stiffness and abrogated by GSK219 or TRPV4 deficiency. **P < 0.01 for cells grown on stiff (8 kPa and 25 kPa) hydrogels vs. soft (1 kPa), ***P < 0.001 for GSK219-treated vs. vehicle treated cells grown on 25 kPa hydrogels; n = 3, t-test. E: both HDFs and MDFs were plated on collagen-coated (10 μg/ml) hydrogels with varying degrees of stiffness (0.5 and 8 kPa). Representative fluorescence micrographs (original magnification, ×40) showing that TRPV4 expression is increased by matrix stiffness (8 kPa vs. 0.5 kPa) in both HDFs and MDFs. F: quantified results are expressed as means ± SE. **P < 0.01, ***P < 0.001 for stiff (8 kPa) vs. soft (0.5 kPa) HDFs. n = 10 cells per condition, 1-way ANOVA.
TRPV4 is required for TGFβ1-induced primary normal dermal myofibroblast differentiation.
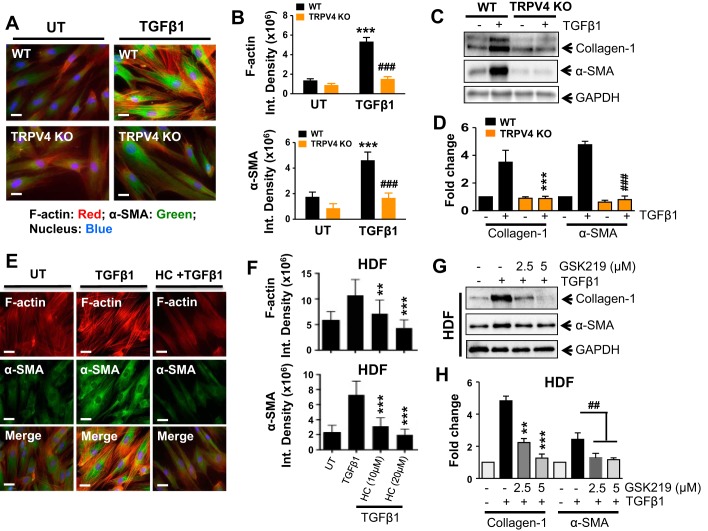
TRPV4 has recently been linked to the differentiation of chondrocytes, osteoclasts, cardiac fibroblasts, and lung fibroblasts (1, 26, 35, 39). To determine whether TRPV4 contributes to dermal myofibroblast differentiation, we compared TGFβ1-induced myofibroblast differentiation (using α-SMA incorporation into stress fibers and expression of collagen-1 protein as an index of myofibroblast differentiation) in primary dermal fibroblasts from TRPV4 KO and congenic WT mice. TGFβ1-induced dermal myofibroblast differentiation was reduced by 60% (n = 15 cells/group; ###P < 0.001) in TRPV4 KO fibroblasts compared with WT (Fig. 4, A and B). In addition, we found that TGFβ1-induced expression of both collagen-1 and α-SMA proteins were reduced in WT MDFs compared with TRPV4 KO (Fig. 4, C and D). These results indicate that TGFβ1-induced dermal myofibroblast differentiation is dependent on TRPV4. Next, we tested whether blocking TRPV4 channel activity with selective small-molecule antagonists abrogates TGFβ1-induced human dermal myofibroblast differentiation. We found that TRPV4 antagonism by its selective antagonist, HC (9, 46), abrogated TGFβ1-induced myofibroblast differentiation by ~60% compared with untreated controls, as shown by α-SMA protein expression, and by incorporation of α-SMA in stress fibers (Fig. 4, E and F; n = 15 cells/condition, **P < 0.01, ***P < 0.001 with or without HC). Furthermore, we demonstrated that TRPV4 antagonism by GSK219, a recently identified novel TRPV4 antagonist (9, 46), abrogated TGFβ1-induced human dermal myofibroblast differentiation by ~70% compared with untreated controls, as shown by expression of collagen-1 and α-SMA protein (Fig. 4, G and H; **P < 0.01, ***P < 0.001; and ##P < 0.01 with or without GSK219). Taken together, these results indicate that TRPV4 calcium channel activity is required for TGFβ1-induced dermal myofibroblast differentiation.
Fig. 4.
TRPV4 is required for TGFβ1-induced dermal myofibroblast differentiation. A: WT and TRPV4 KO MDFs were plated on collagen-coated (10 μg/ml) plastic wells and incubated with or without TGFβ1 (2 ng/ml) for 48 h. Representative fluorescence micrographs (original magnification, ×40) showing that myofibroblast differentiation is reduced in fibroblasts from TRPV4 KO mice (colocalization of α-SMA and F-actin, orange). B: quantification of photomicrographs from A using ImageJ software. Results are expressed as means ± SE ***P < 0.001 for TGFβ1-treated WT cells vs. untreated (UT), and ###P < 0.001 for TRPV4 KO vs. WT cells treated with TGFβ1; n = 15 cells per condition, 1-way ANOVA. C: representative immunoblots showing TGFβ1-induced expression of collagen-1, α-SMA, and GAPDH (control) in WT and TRPV4 KO MDFs. D: quantitation of results from C. ***P < 0.001, ###P < 0.01 for respective WT and TRPV4 KO cells treated with TGFβ1; n = 3, t-test. E: TRPV4 antagonists, HC, blocks TGFβ1-induced HDF differentiation. F: quantified results are expressed as means ± SE **P < 0.01, ***P < 0.001 for TGFβ1 vs. inhibitor (HC) plus TGFβ1-treated HDFs; n = 15 cells per condition, 1-way ANOVA. G: representative immunoblots showing TGFβ1-induced expression of collagen-1, α-SMA, and GAPDH (control) in HDFs with or without TRPV4 inhibitor, GSK219. H: quantitation of results from E. **P < 0.01, ***P < 0.001, ##P < 0.01 vs. respective TGFβ1 only; n = 3, t-test.
Matrix stiffness-induced dermal myofibroblast differentiation is dependent on TRPV4 channels.
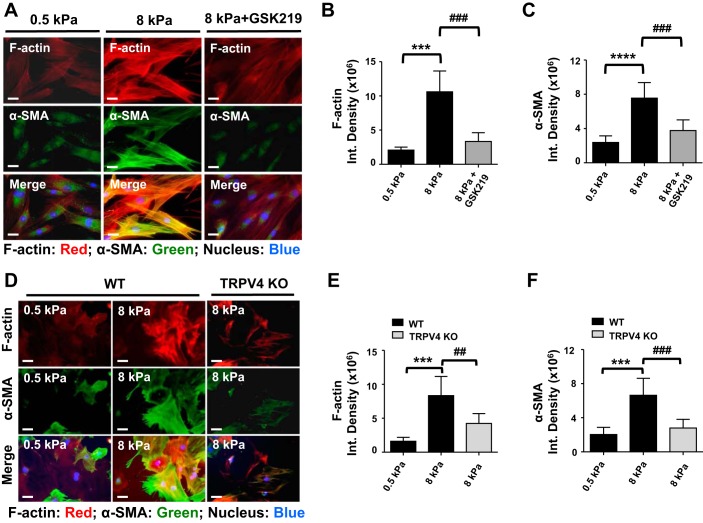
In the context of tissue fibrosis, increased matrix stiffness is well documented, and numerous studies have shown that changes in matrix rigidity can modulate many cellular functions including myofibroblast differentiation (14, 24, 48, 51, 52). However, the molecular mechanism by which matrix stiffness-induced mechanotransduction signals participate in myofibroblast differentiation has remained elusive. To determine whether TRPV4 is involved in matrix stiffness-induced dermal myofibroblast differentiation, we compared myofibroblast differentiation potential of both HDFs and MDFs grown on collagen-coated hydrogels of various degrees of stiffness. We observed increased myofibroblast differentiation when fibroblasts were grown on 8 kPa hydrogels (pathophysiological stiffness comparable to fibrotic lung tissue) (25, 44, 48) compared with 0.5 kPa (normal lung tissue) as determined by α-SMA expression and its incorporation into stress fibers. We found that blocking TRPV4 channels with GSK219 in HDFs (Fig. 5, A–C), or genetic deletion of TRPV4 in MDFs (Fig. 5, D–F), abrogated the capacity of a stiff matrix to induce myofibroblast differentiation. These results indicate that TRPV4-dependent Ca2+ influx is required for matrix stiffness-dependent myofibroblast differentiation.
Fig. 5.
TRPV4 is required for matrix stiffness-induced dermal myofibroblast differentiation. A: HDFs were plated (10,000 cells per well) on collagen-coated (10 μg/ml) hydrogels with varying degrees of stiffness (0.5 and 8 kPa) for 48 h under vehicle-treated or GSK219-treated (5 μM) conditions. TRPV4 antagonist, GSK219, blocks matrix stiffness-induced human dermal myofibroblast differentiation. Representative photomicrographic images are shown for α-SMA (green), F-actin (red), and nucleus (blue). Original magnification, ×40. B and C: quantified results are expressed as means ± SE ***P < 0.001, ****P < 0.0001 for stiff (8 kPa) vs. soft (0.5 kPa) HDFs without GSK219, and ###P < 0.001 for stiff (8 kPa) vs. stiff (8 kPa) with GSK219; n = 15 cells per condition, 1-way ANOVA. D: WT and TRPV4 KO MDFs were plated on collagen-coated (10 μg/ml) hydrogels with varied stiffness (0.5 and 8 kPa) for 48 h. Representative fluorescence micrographs (original magnification, ×40) showing that myofibroblast differentiation is reduced in fibroblasts from TRPV4 KO mice (colocalization of α-SMA and F-actin, orange). E and F: quantification of photomicrographs from D using ImageJ software. Results are expressed as means ± SE. ***P < 0.001 for soft (0.5 kPa) WT vs. stiff (8 kPa) WT cells, and ##P < 0.01, ###P < 0.001 for stiff (8 kPa) WT vs. stiff (8 kPa) TRPV4 KO cells; n = 15 cells per condition, 1-way ANOVA.
TRPV4 modulates profibrotic TGFβ1 actions in a Smad-independent but PI3K-AKT-dependent manner.
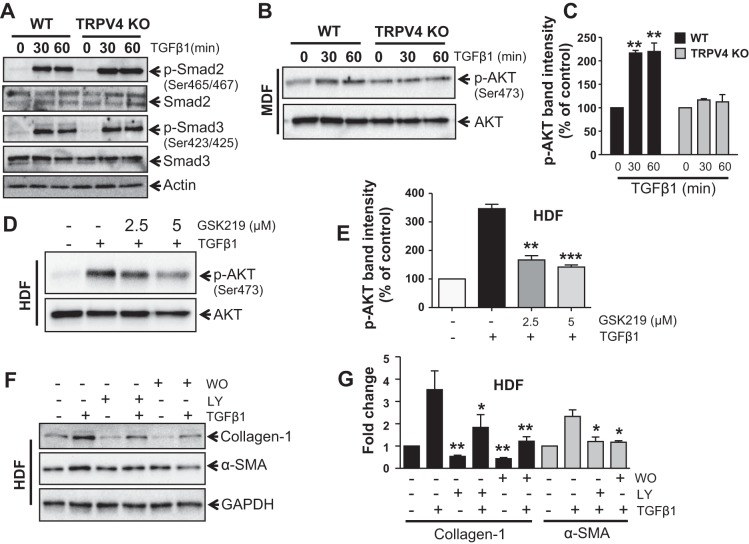
Given that the profibrotic functions of TGFβ1 are regulated by both Smad-dependent and independent signals (2, 4, 55), we assessed the capacity of TRPV4 activity to modulate TGFβ1-induced phosphorylation of Smad2/3 in HDFs. Treatment with TGFβ1 caused an increase in phosphorylation of Smad2/3 as expected, but this response was not inhibited in TRPV4 KO MDFs (Fig. 6A). However, we found that PI3K-AKT signal, a non-Smad pathway, is involved in dermal myofibroblast differentiation induced by TGFβ1 (Fig. 6, B–G). We examined the role of TRPV4 in PI3K activation in dermal fibroblasts. We found that in TGFβ1-stimulated MDFs (Fig. 6, B and C) or HDFs (Fig. 6, D and E) activation of PI3K (as determined by the phosphorylation status of AKT) was reduced ~2- to 3-fold by the loss of TRPV4 activity (TRPV4 KO fibroblasts or GSK219-pretreated HDFs). We also found that in TGFβ1-stimulated HDFs (Fig. 6, F and G) expression level of α-SMA and collagen-1 protein was attenuated by 2- to 3-fold in the presence of PI3K inhibitors, LY or WO. In this study we found that both LY and WO inhibited basal expression level of collagen-1 protein in HDFs. All together these results suggest that TRPV4 activity may regulate TGFβ1-dependent myofibroblast differentiation independent of Smad2/3 activation.
Fig. 6.
TRPV4 activity potentiates profibrotic TGFβ1 signals via Smad2/3-independent manner. WT and TRPV4 KO MDFs were incubated with or without TGFβ1 (2 ng/ml) for 0, 30, and 60 min. A: representative immunoblots showing TGFβ1-induced phosphorylation of Smad2/3 in fibroblasts from both WT and Trpv4 KO mice. Experiments were repeated 3 times. B: representative immunoblots of MDFs (WT and TRPV4 KO) lysates show ~2-fold increase of TGFβ1-induced phosphorylation of AKT (p-AKT). Total AKT was used as loading control. C: quantitation of results from B. **P < 0.01 for WT vs. TRPV4 KO cells treated with TGFβ1; n = 3, t-test. D: immunoblots showing TGFβ1-induced p-AKT in HDFs with or without TRPV4 inhibitor, GSK219. E: quantitation of results from D. **P < 0.01, ***P < 0.001 for cells treated with GSK219 vs. no GSK219; n = 3, t-test. F: immunoblots showing TGFβ1-induced expression of collagen-1, α-SMA, and GAPDH (control) in HDFs with or without PI3K inhibitors LY294002 (LY) or Wortmannin (WO). G: quantitation of results from F. *P < 0.05, **P < 0.01 for untreated vs. cells treated with LY or WO for collagen-1 and α-SMA, respectively; n = 3, t-test.
DISCUSSION
The key findings of this study are 1) functional TRPV4 calcium channels are expressed in primary normal human and murine dermal fibroblasts, 2) TRPV4-mediated Ca2+ influx is upregulated in dermal fibroblasts exposed to both TGFβ1 and pathophysiological matrix stiffness, and 3) TRPV4-dependent Ca2+ influx potentiates TGFβ1-induced dermal myofibroblast differentiation under conditions of matrix stiffness comparable to fibrotic tissue. Furthermore, we provide evidence that TRPV4 inhibition by genetic deficiency or pharmacologic antagonism abrogates TGFβ1-induced phosphorylation of AKT, but not Smad2/3 in both human and mouse dermal fibroblasts.
TRPV4 proteins are ubiquitously expressed in various cell types and tissues, and TRPV4 activity is known to be regulated by both mechanical and soluble factors (9, 13). Given the existence of other TRP family members, it is remarkable that mice deficient only in TRPV4 exhibit modified vasodilatory responses, osmosensing, neurological function, and bone development, and develop ventilation-induced lung edema (3, 8–10, 13, 18, 31, 46). A recent study by our group showed that TRPV4 null mice are protected from developing bleomycin-induced lung fibrosis (39). Many human diseases including skeletal and nerve disorders have been linked to mutations in TRPV4 as a result of gain- or loss-of-function responses (3, 8, 18). Although the importance of the role of TGFβ1, matrix stiffness, and mechanosignaling in dermal myofibroblast differentiation and fibrosis has been recognized (53), the role of TRPV4 mechanosensing in dermal myofibroblast differentiation has not been previously reported. In the current study, we found that TRPV4 protein expression and its channel activity (Ca2+ influx) were upregulated by chronic TGFβ1 exposure in dermal fibroblasts. These results suggest a potential mechanism by which activity of TRPV4 channel can be augmented during scleroderma pathogenesis. Intriguingly, there are emerging data supporting a role for Ca2+-initiated signals in myofibroblast differentiation and function (11, 34); however, the identity of the Ca2+ channel involved, and the mechanisms by which Ca2+ signals promote these effects, are poorly understood. It has been shown that TGFβ1 stimulation of lung fibroblasts can generate an oscillating calcium signal, which is dependent on ER pumps/receptors and on TGFβR kinase activity (34). However, we found that TRPV4-dependent Ca2+ influx in dermal fibroblasts was uniphasic and persistent (>3 min), and was independent of ER pumps/receptors and of TGFβRI kinase activity. These results are consistent with the reported TRPV4-dependent Ca2+ signal in human lung fibroblasts, and suggest that mobilization of internally released Ca2+ is not required for TRPV4-elicited cytoplasmic Ca2+ influx in dermal fibroblasts. Both in vitro and in vivo studies have provided substantial evidence on the contribution of TGFβ1 signals in promoting fibrosis, and in particular, myofibroblast differentiation (5, 15, 36, 40, 47, 48). However, a growing body of data now supports a role for both a mechanical signal, e.g., matrix stiffness, and a biochemical signal, e.g., TGFβ1, in optimal myofibroblast differentiation (14, 25, 48, 51, 52). Furthermore, it is recently well recognized that the stiffened ECM in various fibrotic diseases including scleroderma may augment activation of fibroblasts and subsequent generation of myofibroblasts by chronically releasing active TGFβ1 (14). Conversely, TGFβ1-dependent activation of fibroblasts and generation of contractile and synthetic myofibroblasts can cause increases in matrix stiffness and mechanical responses of the ECM (14, 25, 48, 51, 52). These observations are relevant to the pathophysiology of wound healing and fibrosis, as injuries that trigger fibrotic responses to increase tissue stiffness may subsequently influence overall differentiation of fibroblasts into myofibroblasts in a positive feed-forward manner. The question of how different signaling events, such as matrix stiffness and TGFβ1, might cooperate and/or integrate to affect myofibroblast differentiation remains to be determined.
The elasticity (Young’s modulus) of most plastics and glass used for cell culture is around 1 GPa and 70 GPa, respectively. However, the Young’s modulus of mammalian cells (e.g., fibroblasts) is ~1 kPa. Therefore, the natural cell environment is ~1,000,000 times more elastic than cell culture vessels. Although in vitro studies using cells grown on matrix protein-coated plastic or glass surfaces with infinite stiffness have provided important insights regarding fibroblast differentiation, these approaches have serious limitations with regard to recapitulating the physiological tissue context and matrix stiffness. In our strategy, cells were plated on collagen-coated polyacrylamide gels with physiologically relevant stiffness, which enabled us to decipher the effect of either normal (soft) or fibrotic (stiff) dermal tissue matrix on TRPV4-dependent fibroblast differentiation. We investigated whether TRPV4-integrated mechanical (matrix stiffness) and soluble (TGFβ1) signals mediate dermal myofibroblast differentiation by utilizing collagen-coated polyacrylamide gels of varying stiffness (30). We found that the TRPV4 channel activity (agonist-induced Ca2+ influx) was potentiated when cells were plated on stiffer matrices within the pathophysiological range seen in diseased/fibrotic dermal tissue. The inhibition of TRPV4 channel activity (by genetic deficiency or pharmacological antagonism by three selective TRPV4 antagonists) significantly abrogated TGFβ1-induced enhancement of myofibroblast differentiation when cells were plated on collagen-coated plastics. These data demonstrated that dermal myofibroblast differentiation was sensitive to TRPV4 channel activity. Importantly, inhibition of TRPV4 function in human dermal fibroblasts by selective pharmacological antagonists, or in primary dermal fibroblasts isolated from TRPV4 null mice, resulted in the loss of ability of these cells to undergo TGFβ1-induced myofibroblast differentiation on collagen-coated hydrogels of pathophysiologically relevant stiffness. These results demonstrated that TRPV4 function was required in this process. The results of this study suggest a pathogenic sequence of profibrotic signaling in scleroderma that integrates mechanical (matrix stiffness) and biochemical soluble signals (TGFβ1) to induce myofibroblast differentiation.
TGFβ1 utilizes both canonical (Smad) and noncanonical (non-Smad) intracellular signaling pathways to modulate downstream cellular responses (2, 4, 55). Here, we provide evidence that absence of TRPV4 function (loss of expression or loss of Ca2+ channel activity) does not affect TGFβ1-induced Smad2/3 phosphorylation in dermal fibroblasts. However, in TGFβ1-stimulated human or mouse dermal fibroblasts, activation of PI3K (as determined by the phosphorylation status of AKT) was reduced ~2-fold by the absence of TRPV4 activity, suggesting that the mechanism by which profibrotic TGFβ1 signaling in dermal fibroblasts is modified by TRPV4 may be through noncanonical pathways. Importantly, inhibition of PI3K function in human dermal fibroblasts by selective inhibitors resulted in reduced expression of collagen-1 protein under both basal and TGFβ1-induced conditions. These results all together suggest a possible role of a feed-forward loop involving PI3K and TRPV4 signaling in TRPV4 sensitization, which could be a critical component of pathogenic fibroblast signaling (20).
Furthermore, utilizing both plastic wells and variable stiffness hydrogels, we showed that inhibition of TRPV4 activity abrogated the extensive actin polymerization otherwise seen upon TGFβ1-induced myofibroblast differentiation. In view of the fact that TGFβ1-induced actin polymerization facilitates MRTF-A release from actin monomers, thereby allowing for MRTF-A nuclear translocation, which induces α-SMA transcription during lung myofibroblast differentiation (6, 41), it will be interesting to determine if the mechanism by which TRPV4 potentiates TGFβ1-induced dermal myofibroblast differentiation is through actin polymerization-dependent nuclear translocation of MRTF-A. Recently, Seth et al. (42) revealed that TRPV4 activation protects against nonalcoholic fatty liver disease, thus alerting against the consequences of global blocking of TRPV4 that can promote hepatotoxicity. Therefore, a suitable balanced approach will be required to inhibit TRPV4 by systemic application of TRPV4 blockers to treat fibrotic or other disorders.
In summary, we report a novel role of TRPV4 in dermal myofibroblast differentiation. We show that TRPV4 calcium channel activity modulates profibrotic TGFβ1 actions in a Smad-independent manner, and augments myofibroblast differentiation. In addition, our data show that TRPV4-elicited Ca2+ influx and myofibroblast differentiation are sensitized through interactions of cells with a matrix whose stiffness lies within the pathophysiological range. Importantly, TRP channel-targeted small molecule inhibitors are currently in preclinical or early phase clinical trials for other disorders (33). Since this work identifies a previously unrecognized reciprocal functional link between TRPV4 activation and TGFβ1 signals in dermal fibroblasts, results of this work may lead to a novel therapeutic approach to the treatment of scleroderma and wound healing diseases.
GRANTS
This study was supported by the American Heart Association (AHA 13SDG17310007), Startup, and Maryland Agricultural Experiment Station (MAES) Grant from University of Maryland to S. O. Rahaman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.S., R.G., M.M., J.C., and S.O.R. performed experiments; S.S., R.G., M.M., J.C., and S.O.R. analyzed data; S.S. and S.O.R. prepared figures; S.S. and S.O.R. drafted manuscript; S.S., R.G., M.M., J.C., K.Y.L., D.X.Z., and S.O.R. approved final version of manuscript; K.Y.L., D.X.Z., and S.O.R. interpreted results of experiments; K.Y.L., D.X.Z., and S.O.R. edited and revised the manuscript.
REFERENCES
- 1.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol : 45–52, 2013. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov : 790–811, 2012. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auer-Grumbach M, Olschewski A, Papić L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Fröhlich E, Bálint Z, Tang B, Strohmaier H, Lochmüller H, Schlotter-Weigel B, Senderek J, Krebs A, Dick KJ, Petty R, Longman C, Anderson NE, Padberg GW, Schelhaas HJ, van Ravenswaaij-Arts CMA, Pieber TR, Crosby AH, Guelly C. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet : 160–164, 2010. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors : 196–202, 2011. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelino FV, Varga J. Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy. Curr Opin Rheumatol : 607–614, 2014. doi: 10.1097/BOR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 6.Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol : 6597–6608, 2003. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RW IV, Taylor AB, Wang F, Guilak F, Liedtke W. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain : 1295–1304, 2013. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng H-X, Klein CJ, Yan J, Shi Y, Wu Y, Fecto F, Yau H-J, Yang Y, Zhai H, Siddique N, Hedley-Whyte ET, Delong R, Martina M, Dyck PJ, Siddique T. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet : 165–169, 2010. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol : 2–17, 2010. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA : 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res : 2390–2401, 2010. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Wu L, O’Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem : 27129–27137, 2003. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Elias A, Mrkonjić S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol : 293–319, 2014. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B. Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep : 120–126, 2009. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 15.Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis--a lethal component of systemic sclerosis. Nat Rev Rheumatol : 390–402, 2014. doi: 10.1038/nrrheum.2014.53. [DOI] [PubMed] [Google Scholar]
- 16.Kanju P, Chen Y, Lee W, Yeo M, Lee SH, Romac J, Shahid R, Fan P, Gooden DM, Simon SA, Spasojevic I, Mook RA, Liddle RA, Guilak F, Liedtke WB. Small molecule dual-inhibitors of TRPV4 and TRPA1 for attenuation of inflammation and pain. Sci Rep : 26894, 2016. doi: 10.1038/srep26894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol : 1495–1502, 2006. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 18.Lamandé SR, Yuan Y, Gresshoff IL, Rowley L, Belluoccio D, Kaluarachchi K, Little CB, Botzenhart E, Zerres K, Amor DJ, Cole WG, Savarirayan R, McIntyre P, Bateman JF. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat Genet : 1142–1146, 2011. doi: 10.1038/ng.945. [DOI] [PubMed] [Google Scholar]
- 19.Leddy HA, McNulty AL, Lee SH, Rothfusz NE, Gloss B, Kirby ML, Hutson MR, Cohn DH, Guilak F, Liedtke W. Follistatin in chondrocytes: the link between TRPV4 channelopathies and skeletal malformations. FASEB J : 2525–2537, 2014. doi: 10.1096/fj.13-245936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, Liedtke W. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect : 784–793, 2011. doi: 10.1289/ehp.1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc : 799–810, 2008. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liedtke W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann NY Acad Sci : 42–52, 2008. doi: 10.1196/annals.1418.012. [DOI] [PubMed] [Google Scholar]
- 23.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA : 13698–13703, 2003. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA , Suppl 2: 14531–14536, 2003. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol : 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, Bouillon R, Nilius B, Carmeliet G. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab : 257–265, 2008. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol : 435–442, 2010. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNulty AL, Leddy HA, Liedtke W, Guilak F. TRPV4 as a therapeutic target for joint diseases. Naunyn Schmiedebergs Arch Pharmacol : 437–450, 2015. doi: 10.1007/s00210-014-1078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalick L, Erfinanda L, Weichelt U, van der Giet M, Liedtke W, Kuebler WM. Transient receptor potential vanilloid 4 and serum glucocorticoid-regulated kinase 1 are critical mediators of lung injury in overventilated mice in vivo. Anesthesiology : 300–311, 2017. doi: 10.1097/ALN.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 30.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, Tschumperlin DJ. A multiwell platform for studying stiffness-dependent cell biology. PLoS One : e19929, 2011. doi: 10.1371/journal.pone.0019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol : C96–C101, 2003. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- 32.Moore C, Cevikbas F, Pasolli HA, Chen Y, Kong W, Kempkes C, Parekh P, Lee SH, Kontchou N-A, Yeh I, Jokerst NM, Fuchs E, Steinhoff M, Liedtke WB. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci USA : E3225–E3234, 2013. doi: 10.1073/pnas.1312933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov : 601–620, 2011. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee S, Kolb MRJ, Duan F, Janssen LJ. Transforming growth factor-β evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. Am J Respir Cell Mol Biol : 757–764, 2012. doi: 10.1165/rcmb.2011-0223OC. [DOI] [PubMed] [Google Scholar]
- 35.Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, Shiojiri S, Tashiro K, Suzuki Y, Nishimura R, Kuhara S, Sugano S, Yoneda T, Matsuda A. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem : 32158–32167, 2007. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 36.Nagaraja V, Denton CP, Khanna D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology (Oxford) : 1944–1953, 2015. doi: 10.1093/rheumatology/keu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA : 1316–1321, 2014. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paterno J, Vial IN, Wong VW, Rustad KC, Sorkin M, Shi Y, Bhatt KA, Thangarajah H, Glotzbach JP, Gurtner GC. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen : 49–58, 2011. doi: 10.1111/j.1524-475X.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- 39.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, Moran MM, Schilling WP, Tschumperlin DJ, Olman MA. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest : 5225–5238, 2014. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum : 208–219, 2014. doi: 10.1016/j.semarthrit.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Sandbo N, Dulin N. Actin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving function. Transl Res : 181–196, 2011. doi: 10.1016/j.trsl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seth RK, Das S, Dattaroy D, Chandrashekaran V, Alhasson F, Michelotti G, Nagarkatti M, Nagarkatti P, Diehl AM, Darwin Bell P, Liedtke W, Chatterjee S. TRPV4 activation of endothelial nitric oxide synthase resists nonalcoholic fatty liver disease by blocking CYP2E1-mediated redox toxicity. Free Radic Biol Med : 260–273, 2017. doi: 10.1016/j.freeradbiomed.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J : 4453–4461, 2007. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Southern BD, Grove LM, Rahaman SO, Abraham S, Scheraga RG, Niese KA, Sun H, Herzog EL, Liu F, Tschumperlin DJ, Egelhoff TT, Rosenfeld SS, Olman MA. Matrix-driven Myosin II Mediates the Pro-fibrotic Fibroblast Phenotype. J Biol Chem : 6083–6095, 2016. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res : 1123–1130, 2009. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian M-Y, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med : 159ra148, 2012. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 47.Trojanowska M. Noncanonical transforming growth factor beta signaling in scleroderma fibrosis. Curr Opin Rheumatol : 623–629, 2009. doi: 10.1097/BOR.0b013e32833038ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tschumperlin DJ. Fibroblasts and the ground they walk on. Physiology (Bethesda) : 380–390, 2013. doi: 10.1152/physiol.00024.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MAJ. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun : 490–494, 2009. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA : 396–401, 2004. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webster KD, Ng WP, Fletcher DA. Tensional homeostasis in single fibroblasts. Biophys J : 146–155, 2014. doi: 10.1016/j.bpj.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong VW, Longaker MT, Gurtner GC. Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol : 981–986, 2012. doi: 10.1016/j.semcdb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med : 148–152, 2011. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol : 199–210, 2008. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med : 1028–1040, 2012. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension : 532–538, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu MX. TRP Channels. Boca Raton, FL: CRC Press/Taylor & Francis, 2011. [Google Scholar]