Figure 3.
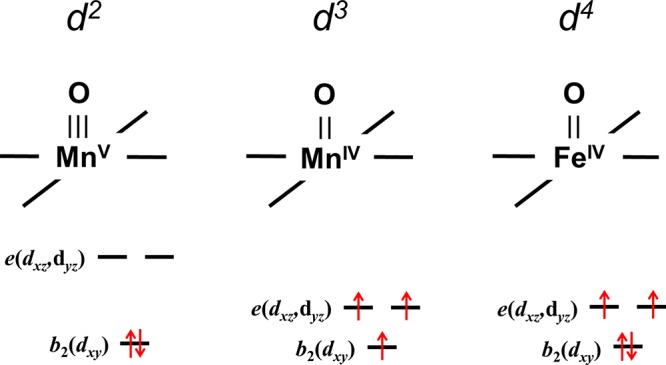
ΔEπ, the energy gap between the eπ*(dxz,dyz) and b2(dxy) orbitals, is a function of the electronic configuration: as electrons are added to eπ*(dxz,dyz), the M–oxo bond distance increases and ΔEπ decreases. The smaller energy gap, coupled with the relief of electron–electron repulsion upon electron unpairing, often produces a high-spin S = 3/2 ground state in the d3 configuration. The reduced M–oxo π-bond order in d3 and d4 configurations increases the basicity of M–oxo complexes.
