Abstract
Context
Progesterone (P) resistance is a hallmark of endometriosis, but the underlying mechanism(s) for loss of P sensitivity leading to lesion establishment remains poorly understood.
Objective
To evaluate the association between Notch-1 signaling activation and P resistance in the progression of endometriosis.
Design
Case control study; archived formalin-fixed, paraffin-embedded tissues.
Setting
University hospitals (United States, Taiwan).
Patients
Women with endometriosis; human endometrial stromal cell line (HESC).
Intervention
Eutopic endometria (EU) and ectopic lesions (ECs) were collected from surgically diagnosed patients. Archived tissue sections of EU and ECs were identified. HESCs were treated with N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) and valproic acid (VPA) to, respectively, suppress and induce Notch-1 activation.
Outcome Measures
Tissues were analyzed for Notch Intra-Cellular Domain 1 (NICD1) and progesterone receptor (PGR) protein expression by immunohistochemistry and for transcript levels of NICD1 target genes HES1, PGR, and PGR-B by quantitative reverse transcription polymerase chain reaction. DAPT- or VPA-treated HESCs with and without P cotreatment were evaluated for cell numbers and for PGR, HES1, and PGR target gene DKK1 transcript levels.
Results
Nuclear-localized stromal NICD1 protein levels were inversely associated with those of total PGR in EU and ECs. Stromal ECs displayed higher HES1 and lower total PGR and PGR-B transcript levels than EU. In HESCs, DAPT reduction of NICD1 decreased cell numbers and increased PGR transcript and nuclear PGR protein levels and, with P cotreatment, maintained P sensitivity. Conversely, VPA induction of NICD1 decreased PGR transcript levels and, with P cotreatment, abrogated P-induced DKK1 and maintained HES1 transcript levels.
Conclusions
Aberrant Notch-1 activation is associated with decreased PGR that contributes to P resistance in endometriosis.
Keywords: ectopic lesions, endometriosis, NICD1, Notch-1, progesterone receptor
Notch-1 activation is associated with loss of progesterone receptor expression in ectopic lesions of endometriotic patients and with loss of progesterone sensitivity in endometrial stromal cells.
Endometriosis is an estrogen-dependent inflammatory disorder defined by growth of endometrial fragments outside of the uterine cavity. With its comorbid conditions of infertility, pregnancy loss, chronic pain [1–4], and increased risks for ovarian cancer [5] and coronary heart disease [6], endometriosis affects 6% to 10% of all reproductive-aged women and has an annual economic impact of >$60 billion in the United States alone. Genomic and proteomic studies have identified many molecules that differed in expression between ectopic lesions (ECs) and eutopic endometria (EU) from diagnosed patients and with those of women without disease [7–9]. Nevertheless, the causes and mechanisms of lesion establishment remain largely undefined. Understanding the complex regulatory network underlying endometriosis is requisite for its prevention, diagnosis, and effective treatment.
Progesterone (P) resistance is a major component in the constellation of disorders that characterize endometriosis [1, 3, 10, 11]. Using an immunocompetent mouse model of endometriosis recently established by our group [12], we found that activation of Notch-1 signaling [measured by increased levels of Notch Intra-Cellular Domain 1 (NICD1)] is associated with decreased expression of progesterone receptor (PGR) in ECs. This is consistent with the report that reduced PGR expression resulted from aberrant activation of Notch-1 signaling in mouse uteri [13]. In that study, the inverse association between Notch-1 activation and PGR expression was linked to infertility, but a potential link with endometriosis was not investigated. Intriguingly, an earlier report from the same group showed that EU of women with endometriosis had decreased Notch-1 signaling that led to impaired decidualization [14], a condition associated with P resistance [15]. These findings suggest that Notch-1 function is endometrial cell context-dependent, analogous to that demonstrated in many cancer types [16, 17].
Current management of endometriosis is based on pharmacological treatments and surgical intervention [18, 19]; however, the efficacy of clinically used drugs is limited and the recurrence rate for endometriosis is ~50% within 3 years after surgery or cessation of treatment. In cancers, increased cell proliferation, survival, and inflammation and decreased steroid hormone responsiveness—all features of endometriotic lesions—are associated with Notch-1 activation [20, 21]. Mouse models of human cancers and clinical trials for hormone-dependent human cancers (e.g., breast, prostate) have demonstrated that use of pharmacological inhibitors of Notch-1 signaling in conjunction with antitumor drugs can inhibit tumor growth with some success [22–24]. Because blockage of Notch-1 activation may constitute a unique intervention for clinical management of endometriosis, studies to further understand the putative link between Notch signaling and endometriosis are warranted.
To address the relationship between Notch-1 activation and P resistance in human endometriosis, we compared EU and ECs for expression levels of PGR and Notch-1 signaling effector (NICD1), ligand [Jagged 2 (JAG2)], and gene target [Hairy and Enhancer of Split-1 (HES1)] and used an immortalized, P-responsive HESC [25] treated with pharmacological modifiers of Notch-1 signaling to assess effects on PGR and PGR target [Dickkopf-related protein 1 (DKK1)] expression. Our data provide evidence to functionally link Notch-1 activation with diminished P sensitivity via a reduction in PGR expression.
1. Materials and Methods
A. Tissue Samples (UAMS Cohort)
Archival formalin-fixed, paraffin-embedded paired endometrial EU and ECs were identified by our team pathologist (C.M.Q.) from interrogation of Department of Obstetrics and Gynecology pathology records (2011 to 2013), following protocols approved by the Institutional Review Board of the University of Arkansas for Medical Sciences (UAMS). Sections were retrieved for women (n = 5) that met the selection criteria (premenopausal, peritoneal endometriosis). Endometriosis stage was classified based on the American Society of Reproductive Medicine guidelines [26]. Tissue sections were processed for evaluation of NICD1 and PGR expression by immunohistochemistry (see below).
B. Study Population and Tissues (Taiwan Cohort)
Endometrial tissue (EU) and EC lesions from nonpregnant women with surgically diagnosed endometriosis were obtained during laparoscopy or surgeries performed at the Department of Obstetrics and Gynecology of the National Cheng Kung University Hospital (Tainan, Taiwan), following protocols approved by the Institution’s Review Board. Participants signed informed consent and were without any hormonal treatments for 6 months prior to surgery. Nonpaired EU (n = 11) and EC (n = 8) samples were obtained in the first set of tissue collection, while the second set comprised of paired EU and EC samples (n = 6). Data available for the participants included endometriosis stage following established classification [26], menstrual cycle phase at collection, age and body mass index. All lesions listed in Supplemental Table 2 were peritoneal endometriosis whereas two of the six lesions in Supplemental Table 3 were classified as endometriomas. Tissues were paraffin embedded and processed for immunostaining with anti-NICD1 antibodies (see below).
C. Immunohistochemistry
Sections (5 μm) of formalin-fixed, paraffin-embedded tissues were dewaxed with xylene, rehydrated through a graded alcohol series, and subjected to antigen retrieval in citrate buffer (10 mM, pH 6.0) with boiling for 15 minutes. Sections were treated with 3% hydrogen peroxide to quench endogenous peroxidase activity and incubated in blocking reagent (Vectastain ABC Kit; Vector Laboratories Inc., Burlingame, CA) for 1 hour. The antibodies [Research Resource Identifier (RRID), www.antibodyregistry.org] and their respective working dilutions (used at incubation conditions of 4°C; 16 to 24 hours) are as follows: (1) rabbit anti-human Notch 1 (RRID:AB_1977387, 1:500) [27]; (2) rabbit anti-human PGR (RRID:AB_2164331, 1:200) [28]; (3) rabbit anti-human ESR1 (RRID:AB_631470, 1:200) [29]; (4) rabbit anti-human ESR2 (RRID:AB_310195, 1:200) [30]; and (5) rabbit anti-Ki67 monoclonal (RRID:AB_302459, 1:100) [31]. Sections were subsequently washed in PBS, incubated with biotinylated anti-rabbit secondary antibodies (Vector Laboratories Inc.) for 1 hour at room temperature and after additional washings, were treated with 3,3′-diaminobenzidine tetra-hydrochloride (Dako Inc., Carpenteria, CA) followed by counterstaining with hematoxylin. Normal rabbit IgG was used as a negative control for staining. For the UAMS sample cohort, the stained sections were captured by a digital pathology whole slide scanner (Aperio Image Scope; Leica Biosystems, Buffalo Grove, IL), and the number of nuclear-stained stromal cells in three to four random visual fields for each tissue section was counted. Data are expressed as a percentage of the number of nuclear-stained cells, relative to the total number of cells counted. For the Taiwan sample cohort, NICD1-immunostained cells in EU (n = 17) and ECs (n = 14) were visualized under a light microscope and evaluated in four to five random fields per slide per tissue sample. Histoscore (H-score) was calculated by a semiquantitative assessment of staining intensities in positively stained cells on a scale of 0-3 (0 = none, weak = 1, moderate = 2, strong = 3) performed by two individuals blinded to the study.
D. Primary Stromal Cell Isolation
Primary stromal cells from paired EU and ECs were isolated and evaluated for transcript levels of Notch-1 signaling components HES1 and JAG2 and of total PGR and PGR-B isoform. The isolation of stromal cells and confirmation of purity (>99%) by immunostaining with vimentin (stromal cell–specific) and cytokeratin (epithelial cell–specific) antibodies followed previously described protocols [32, 33].
E. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated using TRIsure (Taiwan-Bioline USA Inc., Boston, MA) or TRIzol (UAMS-Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Total RNA (250 ng) was reverse transcribed to cDNA (iScript cDNA Synthesis Kit; Bio-Rad Laboratories, Hercules, CA) and used for SYBR green-based real-time PCR (Taiwan: Roche Diagnostics, San Francisco, CA; UAMS: Bio-Rad Laboratories), following our previously published protocols [12, 32–34]. Intron-flanking primers were designed to eliminate genomic DNA amplification using the Primer Express software (Applied Biosystems Inc., San Francisco, CA) and were obtained from Integrated DNA Biotechnologies, Inc. (Coralville, IA). Target mRNA abundance was normalized to 18S rRNA (for HES1, JAG2, PGR, and PGR-B in EU and ECs) or TATA-Binding Protein mRNA (for HES1, DKK1, and PGR in HESCs).
F. Cell Culture and Treatments
The HESC (ATCC CRL-4003) was obtained from the American Type Culture Collection (ATCC; Manassas, VA) and was authenticated by the supplier using short tandem repeat DNA profiling. Cells were propagated following procedures described by ATCC and seeded at 5 × 103 cells/well onto 96-well culture plates (Corning Ware, Woodside, NY) for cell counts or 1 × 105 cells/well onto 12-well culture plates (Corning Ware) for gene expression analysis. Cells were cultured to 50% to 70% confluence in 10% charcoal-stripped bovine calf serum (CS-BCS; Invitrogen), synchronized by serum starvation in 2% CS-BCS for 24 hours, and then treated with γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT; Sigma-Aldrich, St. Louis, MO) [35] or histone deacetylase inhibitor valproic acid (VPA; Sigma-Aldrich) [36] in 10% CS-BCS at the concentration indicated for each experiment. Vehicle control treatments were dimethyl sulfoxide (DMSO; Thermo-Fischer, Waltham, MA) for DAPT and PBS for VPA in culture media. Cells were incubated with DAPT or VPA (and corresponding vehicles) for 48 hours prior to cell count assays (Hemocytometer; Thermo-Fischer). In cotreatment studies with P (medroxyprogesterone acetate, Sigma-Aldrich), cells were treated with either DAPT or VPA for 48 hours prior to P treatment (1 μM) for an additional 24 hours. Treatments for cell count assays were performed in quadruplicate and each experiment was repeated three times. To determine the effects of DAPT and VPA alone or in combination with P on gene expression, treatments were performed in triplicate, and each experiment was repeated three times.
G. Immunocytochemistry
HESCs were grown to ~70% confluence on glass coverslips placed in six-well culture dishes and incubated in media supplemented with DAPT (10 μM) or VPA (2 mM) for 48 hours. Treated cells were then fixed in 4% paraformaldehyde for 10 minutes, permeabilized with 0.2% Triton X-100 for 30 minutes, and blocked with 1% goat serum blocking solution (Vector Laboratories Inc.) for 1 hour; all procedures were carried out at room temperature. The cells were incubated with anti-NICD1 (1:200 dilution) or anti-PGR (1:200 dilution) antibodies at 4°C overnight, washed twice with PBS, and then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibodies (1:200 dilution; Thermo-Fischer) for 1 hour at room temperature in a dark room. Cells were treated with 4′,6-diamidino-2-phenylindole (DAPI, 0.3 µM; Vector Laboratories) to stain for nuclei. Fluorescence images were collected using a Cytation 5 Multi-Mode Reader (BioTek Instruments; Winooski, VT). The nuclear distribution (i.e., colocalized DAPI and fluorescein isothiocyanate fluorescence) of NICD1 and of PGR was evaluated from four random fields for each slide at ×200 magnification, with three slides analyzed per treatment group. Data are expressed as a percentage of the numbers of nuclear-stained cells relative to the total number of cells in a given field.
H. Statistical Analyses
Numeric data are presented as mean ± Standard Deviation (SD) and were compared between experimental groups by t test (for two groups) or one-way analysis of variance, followed by Tukey post hoc test (for three or greater groups), using SigmaStat version 3.5 software (SPSS Inc., Chicago, IL). A P value ≤0.05 was considered to be statistically significant.
2. Results
A. Notch-1 Activation and Expression of Endometriosis Biomarkers in ECs
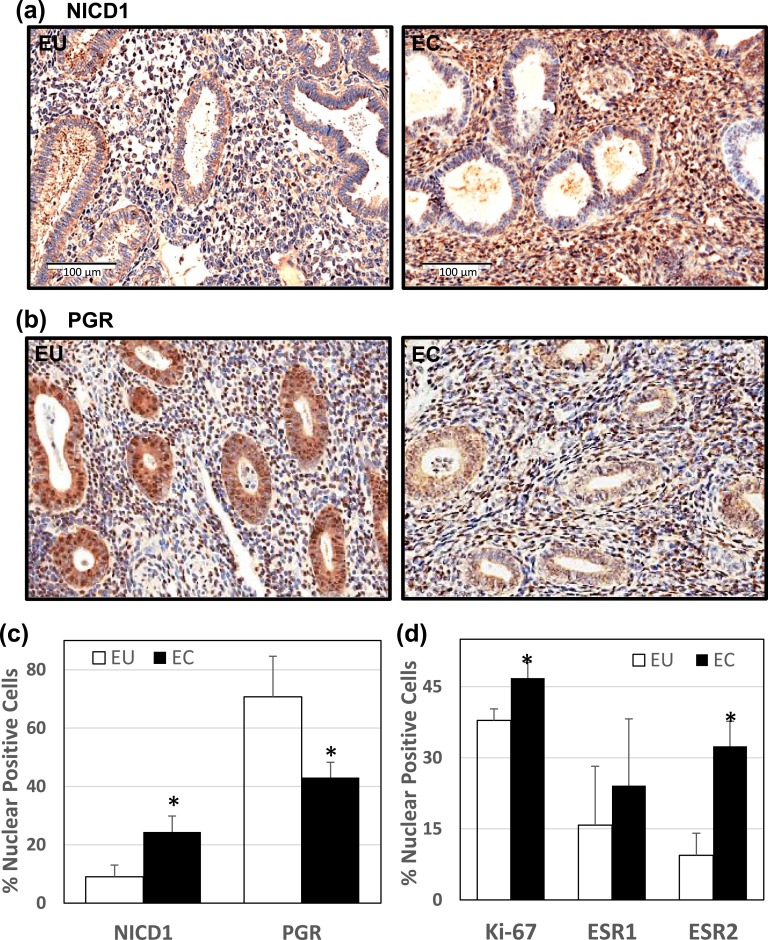
To investigate the potential contribution of Notch-1 signaling to human endometriosis, we evaluated the expression levels of nuclear-localized NICD1, a measure of Notch-1 signaling activation, together with those of PGR and other biomarkers of endometriosis, in paired EU and ECs of women with endometriosis (UAMS cohort). Representative immunostaining images for NICD1 [Fig. 1(a)] and PGR [Fig. 1(b)] are shown. Relative to EU, ECs showed higher and lower percentages of stromal cells with nuclear-localized NICD1 and PGR, respectively [Fig. 1(c)]. Moreover, ECs displayed higher percentages of stromal cells expressing the proliferation marker Ki67 and estrogen receptor-β (ESR2) in nuclei, than EU. However, the percentage of nuclear estrogen receptor-α (ESR1)–expressing cells was comparable between EU and ECs [Fig. 1(d)].
Figure 1.
Expression of NICD1 and PGR in EU vs ECs of women with peritoneal endometriosis. Formaldehyde-fixed, paraffin-embedded sections of paired EU and ECs from the same patient (UAMS cohort) were stained with anti-NICD1 or anti-PGR antibodies. Representative images of (a) NICD1 and (b) PGR immunostaining for EU vs ECs are shown. (c) The percentages of nuclear-localized, immunostained stromal cells for NICD1 and PGR were determined by counting the number of immunopositive nuclei over the total number of cells counted per field. Data (mean ± SD) represent analyses of tissue sections from n = 5 patients per group. For each tissue section, three to four random visual fields were counted. (d) Tissue sections were immunostained with anti-Ki67, anti-ESR1, and anti-ESR2 antibodies and analyzed as in c. Data (mean ± SD) are from n = 5 patients per group. For each tissue section, three to four random visual fields were counted. *P < 0.05 by Student paired t test between stromal EU and ECs.
B. Inverse Association of Notch-1 Signaling and PGR in ECs
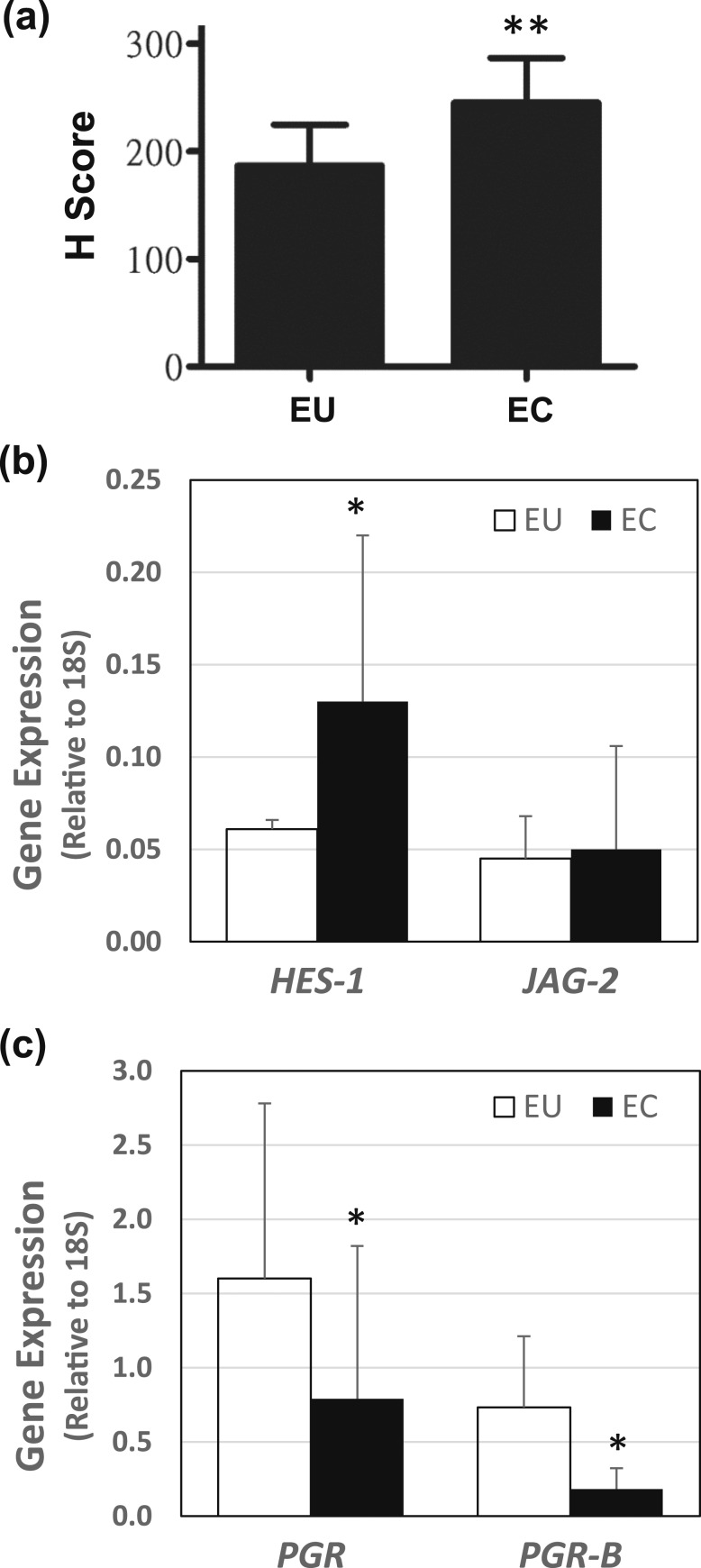
To confirm the induction of Notch-1 signaling in ECs, EU and ECs from women with endometriosis were collected during surgery (Taiwan cohort) and immunostained for NICD1. Staining intensities were semiquantitatively scored using a range of 0 to 3, with highest intensity designated as 3 (strong) > 2 (moderate) > 1 (weak), with no staining indicated as 0. ECs showed higher H-scores than EU for stromal NICD1 [Fig. 2(a)], in agreement with that shown for the UAMS samples (Fig. 1).
Figure 2.
Notch signaling components and PGR expression in EU and ECs. (a) Paraffin-embedded eutopic endometrial tissue (n = 17) and ECs (n = 14) from women with diagnosed endometriosis (Taiwan cohort) were immunostained with anti-NICD1 antibody. Staining scores (0 = none, 1 = weak, 2 = moderate, and 3 = strong) were assigned to immunostained samples. H-scores (mean ± SD) were calculated as described under “Materials and Methods.” **P < 0.0001 by Student unpaired t test between stromal EU and ECs. (b) Stromal cells were isolated from paired EU and ECs of women with endometriosis (n = 6) and subjected to quantitative polymerase chain reaction analyses to quantify Notch signaling components HES1 and JAG2 mRNA levels. (c) Stromal cells were isolated from paired EU and ECs of women with endometriosis (n = 6) and subjected to quantitative polymerase chain reaction analyses to quantify total PGR and PGR-B mRNA levels. For b and c, data (mean ± SD) were normalized to 18S rRNA. *P < 0.05 by Student paired t test between stromal EU and ECs.
Paired EU and ECs collected from another set of women with endometriosis (n = 6) were evaluated for an association between NICD1 activation and PGR expression. Stromal cells were isolated from each tissue sample and assayed (without further culture) for transcript levels of HES1, JAG2, total PGR, and PGR-B. Stromal HES1 transcript levels were higher for ECs than for EU, whereas those for JAG2 did not differ between EU and ECs [Fig. 2(b)]. Total PGR mRNA levels were higher for stromal EU than ECs, and this trend was mimicked by PGR-B [Fig. 2(c)]. However, although total PGR mRNA levels decreased by ~50% in ECs relative to EU, PGR-B mRNA levels in ECs showed a greater degree of reduction (by ~75%) when compared with EU.
C. Pharmacological Modification of Notch-1 Signaling and PGR Expression
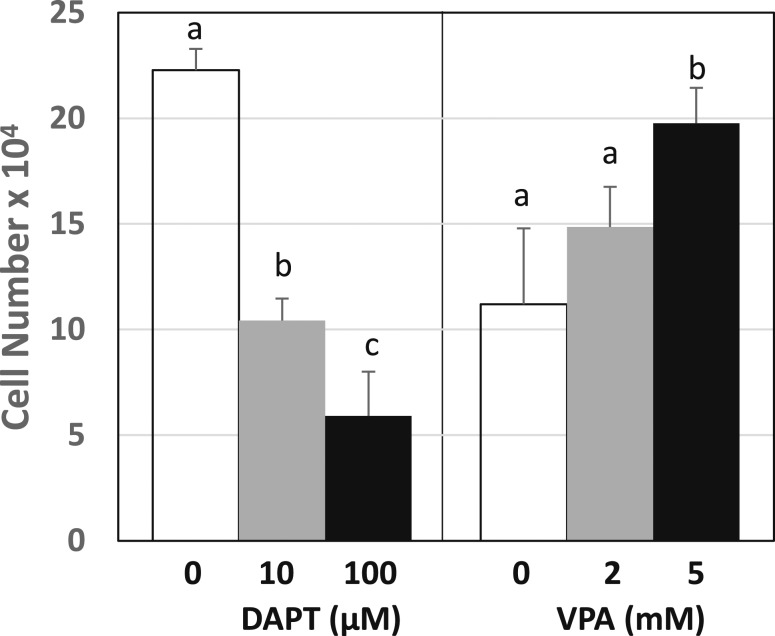
To evaluate the direct effects of Notch-1 signaling activation on endometrial stromal cells, two well-known pharmacological modifiers of Notch-1 signaling were used to alter basal levels of activated Notch-1 in the human endometrial cell line HESC. DAPT, a γ-secretase inhibitor blocks Notch signaling by inhibiting Notch endoproteolysis to its activated form NICD1 [35]. Conversely, the histone deacetylase inhibitor VPA activates the Notch signaling cascade, leading to increased levels of NICD1 [36]. Treatment of HESCs with DAPT or VPA resulted in opposing effects on cell numbers, with DAPT showing a dose-dependent decrease and, conversely, VPA displaying a dose-dependent increase (Fig. 3).
Figure 3.
Pharmacological manipulation of Notch-1 signaling altered cell numbers of HESCs. Cells were treated with DAPT (left panel) or VPA (right panel) and respective controls (DMSO for DAPT; PBS for VPA). Cell numbers were determined using a hemocytometer. Data (mean ± SD) are from three independent experiments (n = 4 wells/experiment). Within each treatment group (DAPT or VPA), means with different superscripts differed at P < 0.05 as determined by one-way analysis of variance.
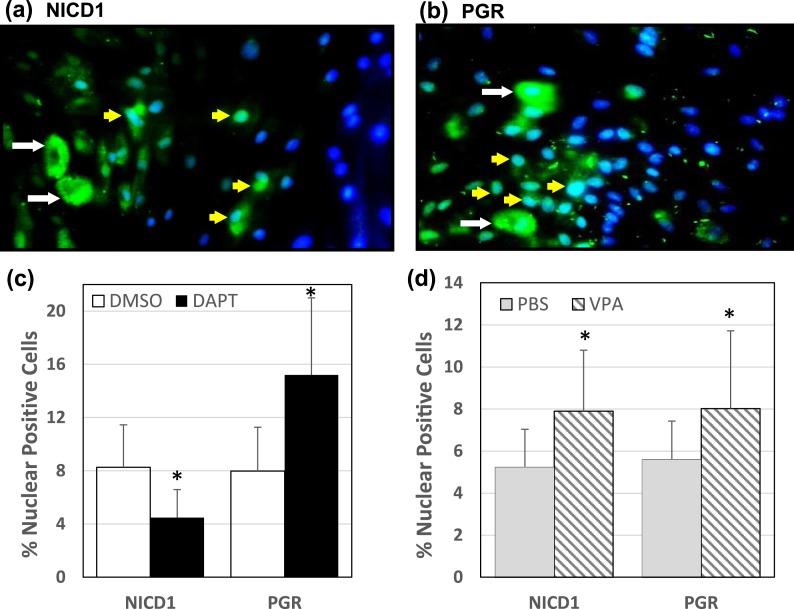
To determine the effects of DAPT and VPA on nuclear NICD1 levels in HESCs and the functional consequences of changes in NICD1 levels, if any, on PGR expression, cells were treated with DAPT (10 µM) or VPA (2 mM) and visualized by immunostaining for NICD1 and PGR in DAPI-stained cells. Representative images of cells treated with DAPT and stained with anti-NICD1 [Fig. 4(a)] and of cells treated with VPA and stained with anti-PGR [Fig. 4(b)] show presence of cells in both cytoplasmic (white arrow) and nuclear (yellow arrow) compartments. The percentages of nuclear-positive cells (i.e., colocalizing with DAPI staining) for NICD1 and for PGR in DAPT-treated [Fig. 4(c)] and VPA-treated [Fig. 4(d)] cells were quantified. DAPT decreased and VPA increased the percentage of cells with nuclear-localized NICD1. Conversely, DAPT increased the percentage of cells with nuclear-localized PGR [Fig. 4(c)]. VPA similarly increased the percentage of cells with PGR localized to the nuclear compartment [Fig. 4(d)]. However, the magnitude of this increase (~40%) was less robust than that attained (~90%) with DAPT treatment [Fig. 4(d) vs Fig. 4(c)].
Figure 4.
Changes in nuclear-localized NICD1 and PGR in HESCs treated with DAPT or VPA. HESCs were treated with DAPT (10 μM), VPA (2 mM), or corresponding vehicle controls (DMSO for DAPT; PBS for VPA) and immunostained with anti-NICD1 and anti-PGR antibodies followed by incubation with fluorescence-conjugated secondary antibodies. Cells were counterstained with DAPI to identify nuclei. (a) Representative picture of anti-NICD1-immunostained and DAPI-counterstained cells after DAPT treatment. (b) Representative picture of anti-PGR-immunostained and DAPI-counterstained cells after VPA treatment. Nuclei were localized by DAPI (blue staining). For a and b, cytoplasmic-localized (green; white arrow) and nuclear-localized (blue-green; yellow arrow) proteins are shown. Nuclear-localized immunopositive cells for NICD1 and PGR were counted in cells treated with (c) DAPT or (d) VPA (and corresponding controls) and expressed relative to the total number of cells analyzed. Data (mean ± SD) are from two independent experiments, with three individual slides evaluated per treatment group per experiment. *P < 0.05 by Student t test between control and treatment groups for NICD1 and for PGR.
D. Changes in P Sensitivity of HESCs With Pharmacological Notch Signaling Modification
To evaluate if changes in nuclear PGR levels observed with DAPT or VPA treatments (Fig. 4) alter P sensitivity of endometrial stromal cells, HESCs were exposed to DAPT or VPA (controls are DMSO and PBS, respectively) for 48 hours and then treated with P (1 μM) for another 24 hours. Collected cells were evaluated for cell numbers and for expression of PGR, HES1, and DKK1.
Relative to DMSO-treated cells, cells treated with DAPT or P alone showed comparable reduction in cell numbers [Fig. 5(a)]. Cells cotreated with DAPT and P (DAPT/P) displayed a reduction in cell numbers greater than with either treatment alone [Fig. 5(a)]. In contrast, VPA treatment (2 mM) did not alter cell numbers [Fig. 5(b)] but, when combined with P (VPA/P), increased cell numbers to levels above those obtained with individual and control treatments.
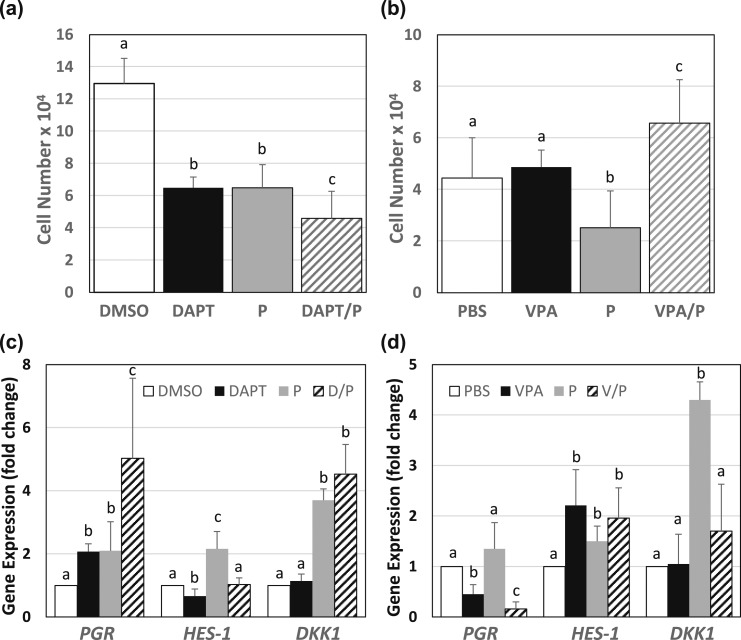
Figure 5.
Altered Notch-1 signaling modified responses of HESCs to progestin. HESCs treated with DAPT (10 μM) or VPA (2 mM) in the presence or absence of medroxyprogesterone acetate (P, 1 μM) (DMSO or PBS as control treatments) were evaluated for (a, b) cell numbers and (c, d) gene expression. Data for cell numbers (mean ± SD) are from three independent experiments (n = 4 wells/experiment). Transcript levels of PGR, HES1, and DKK1 were evaluated by quantitative polymerase chain reaction; TATA-Binding Protein mRNA was used as normalization control. Data (mean ± SD) are expressed as fold change from control cells and were obtained from three independent experiments, with each experiment carried out in duplicate. For a and b, means within each panel showing different superscripts differed at P < 0.05 as determined by one-way analysis of variance. For each gene (PGR, HES1, DKK1) in panels c and d, means with different superscripts differed at P < 0.05 as determined by one-way analysis of variance.
PGR transcript levels were increased by approximately twofold with DAPT or P, relative to DMSO treatment [Fig. 5(c)]. The combination treatments (D/P) increased PGR mRNA levels ~2.5-fold above those elicited with individual treatments. HES1 transcript levels were reduced by DAPT and increased by P treatment alone, whereas the combination treatments (D/P) abrogated the individual treatment effects to control levels. DKK1 transcript levels were increased by P treatment (~3.8-fold) but were unaffected by DAPT alone or when combined with P.
VPA decreased, whereas P tended to increase, PGR transcript levels, whereas the cotreatments of VPA and P (V/P) enhanced the reduction of PGR expression to levels below that of control (PBS) or VPA-treated cells [Fig. 5(d)]. As expected, VPA increased HES1 transcript levels, relative to control cells. P alone increased HES1 gene expression but did not alter VPA’s individual effect in combined treatments. DKK1 transcript levels were highly induced by P treatment and were reduced by VPA and P (V/P) cotreatment to control levels, although VPA alone showed no effect.
3. Discussion
In a previous study [12], we provided evidence in a mouse model to suggest that Notch-1 signaling activation is associated with progression of endometriosis. In the current study, we extended these findings by our analyses of EU and ECs collected from women with confirmed endometriosis at two different university hospitals. Our results demonstrated induction of Notch-1 signaling, assessed by the higher levels of nuclear-localized NICD1 and of HES1 transcript, in stromal cells of ECs relative to EU. Further, we showed a negative association between Notch-1 activation and PGR expression, coincident with increased proliferation (Ki67) and enhanced ESR2 expression, in ECs relative to EU. Finally, we confirmed in an endometrial stromal cell line (HESC), by using pharmacological modifiers of Notch-1 signaling, that P sensitivity is optimally sustained with reduction of Notch-1 activation (by DAPT) and, conversely, is robustly attenuated with continued Notch-1 activation (by VPA), due in part to negative regulation by activated Notch of PGR expression. Our results support a role for chronic Notch-1 signaling activation underlying P resistance in women with endometriosis and suggest that the clinical management of the disease may benefit from stringent control of Notch-1 signaling in endometrial stromal cells.
The contribution of Notch signaling to the initiation and progression of endometriosis in women is likely important, although this remains poorly understood. Herein, we report that Notch-1 activation is higher, coincident with decreased PGR/PGR-B expression in ECs vs EU. This inverse association is consistent with a previous report in mice that aberrant activation of uterine Notch-1 signaling caused hypermethylation of the Pgr gene, resulting in reduction of uterine PGR expression and infertility [13]. An earlier study from the same group demonstrated that expression of several Notch signaling components (NOTCH 1/2, NOTCH ligands JAG2 and Delta-like 4) and NICD1 direct target genes HES5 and HEY1 were lower in endometria of women with, than without, endometriosis [14]. Although NICD1 levels were not reported, the noted increase in Notch-1 signaling in EU relative to normal endometria does not support the inverse correlation between Notch-1 activation and PGR expression reported in their earlier study [13] and in our present study, and the previously reported decrease in PGR expression in EU relative to endometria of women without endometriosis [10, 11, 34]. Taken together, results suggest that Notch-1 activation provides a regulatory node for P signaling that may differ in normal vs pathological cells.
We found that Notch-1 activation in ECs (relative to EU) was associated with increased ESR2 but not ESR1 levels. ECs are characterized by elevated expression of ESR2 relative to ESR1 [11, 37], and ECs generated in mice from ESR2-overexpressing endometrium displayed larger volumes than those from ESR2-null endometrium [37]. ESR2 has marked nongenomic actions in ECs, which include induction of inflammation [37], a known trigger of Notch-1 signaling [38, 39]. Inflammation has also been demonstrated to reduce PGR expression and PGR-mediated transcriptional activity [40]. In vascular endothelium, estrogen-mediated regulation of NICD1 levels was abrogated after silencing ESR2 [41]. Thus, in the context of endometriosis, ESR2’s substantial expression and inflammatory effects may tip the balance toward Notch-1 activation and NICD1 downregulation of PGR expression and signaling.
We used pharmacological agents known to interfere with Notch-1 signaling to determine direct effects of Notch-1 activation (VPA) and inhibition (DAPT) in vitro on established EC lesion parameters (higher cell numbers, lower PGR expression) by using the HESC. We found that DAPT treatment in a progestogenic environment (i.e., with P treatment) reduced cell numbers and increased PGR expression beyond those obtained with individual P or DAPT exposure and maintained robust PGR transcriptional activity (e.g., DKK1 transcript levels) in the HESC. Conversely, VPA/P cotreatment increased cell numbers and abrogated PGR-transcriptional activity, coincident with reduction of PGR expression. Together, these findings demonstrate that P sensitivity of stromal cells is responsive to alterations in NICD1 levels and suggest that Notch-1 is an upstream regulator of the PGR signaling pathway. Further, because Notch signaling is increased by P in uterine stromal cells [42], in agreement with our findings here that P treatment alone increased HES1 transcript levels [Fig. 5(c) and 5(d)], presumably by increasing NICD1, results indicate a requirement for the precise maintenance of the PGR/Notch regulatory network, the disruption of which may underlie some, though not all, uterine disorders.
The coincident increase in the percentage of cells with nuclear-localized PGR and with nuclear-localized NICD1 upon VPA treatment [Fig. 4(d)] is unexpected, given that this was accompanied by a reduction in total PGR and induction of HES1 transcript levels [Fig. 5(d)], respectively. We speculate that the above findings may be related to the differential reduction (PGR-B over PGR-A) and/or the preferential nuclear localization (PGR-A over PGR-B) of PGR isoforms subsequent to Notch-1 activation. We used an anti-PGR antibody recognizing both PGR isoforms in these experiments and, hence, are unable to directly address this issue. Nevertheless, we showed herein a disproportionate decrease in PGR-B transcript levels relative to total PGR transcripts in stroma of EC lesions [Fig. 2(c)]. Moreover, progression to higher endometrial tumor grades was associated with the preferential reduction of focally distributed PGR-B relative to PGR-A [43]. Further studies will address if the latter are similarly observed in the context of endometriosis, using specific anti-PGR-A antibodies. The noted cytoplasmic localization of PGR is not surprising, given its nongenomic actions [44]. Similarly, NICD1 has been localized to membrane, cytoplasmic, and nuclear compartments in many cell types [45], although its detailed compartmental distribution in uterine cells has yet to be described.
Recognized limitations to the current study include the relatively small patient numbers and amounts of tissues, which precluded more extensive analytical analyses, differences in the race/ethnicity of the study population, and variations in the procedures used for tissue identification/collection at the two clinical sites. Despite these, the collective findings established a consistent inverse association between NICD1 and PGR in ECs. In the UAMS sample cohorts, the higher Ki67 and ESR2 protein levels in ECs relative to EU are consistent with their recognized roles as markers of endometriotic lesions. Moreover, the robust differences in HES1, total PGR, and PGR-B isoform transcript levels found between EU and ECs in the Taiwan cohort suggest that the negative association between NICD1 and PGR may be a feature of more advanced (stage III to IV) endometriosis and, hence, of disease progression.
In conclusion, our findings indicate that aberrant Notch-1 activation may underlie the progestin resistance of ECs of women with endometriosis. Further studies with a larger patient population are indicated to support this functional link.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by a Sturgis Foundation Grant (to R.C.M.S.), University of Arkansas for Medical Sciences Barton Funds (to R.C.M.S.), and the National Institutes of Health/National Cancer Institute RO1CA136493 (to F.A.S. and R.C.M.S.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ATCC
American Type Culture Collection
- CS-BCS
charcoal-stripped bovine calf serum
- DAPI
4′,6-diamidino-2-phenylindole
- DAPT
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- DKK1
Dickkopf-related protein 1
- DMSO
dimethyl sulfoxide
- EC
ectopic lesion
- ESR1
estrogen receptor-α
- ESR2
estrogen receptor-β
- EU
eutopic endometria
- HES1
Hairy and Enhancer of Split-1
- HESC
human endometrial stromal cell line
- JAG2
Jagged 2
- NICD1
Notch Intra-Cellular Domain 1
- P
progesterone
- PGR
progesterone receptor
- RRID
Research Resource Identifier
- SD
standard deviation
- UAMS
University of Arkansas for Medical Sciences
- VPA
valproic acid
References and Notes
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127(1):92–100. [DOI] [PubMed] [Google Scholar]
- 3. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glavind MT, Forman A, Arendt LH, Nielsen K, Henriksen TB. Endometriosis and pregnancy complications: a Danish cohort study. Fertil Steril. 2017;107(1):160–166. [DOI] [PubMed] [Google Scholar]
- 5. Wilbur MA, Shih IM, Segars JH, Fader AN. Cancer implications for patients with endometriosis. Semin Reprod Med. 2017;35(1):110–116. [DOI] [PubMed] [Google Scholar]
- 6. Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 8. Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, DePaolo LV, Giudice LC. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fassbender A, Verbeeck N, Börnigen D, Kyama CM, Bokor A, Vodolazkaia A, Peeraer K, Tomassetti C, Meuleman C, Gevaert O, Van de Plas R, Ojeda F, De Moor B, Moreau Y, Waelkens E, D’Hooghe TM. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum Reprod. 2012;27(7):2020–2029. [DOI] [PubMed] [Google Scholar]
- 10. Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74. [DOI] [PubMed] [Google Scholar]
- 11. Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, Thung S, Su EJ, Kim JJ. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heard ME, Simmons CD, Simmen FA, Simmen RCM. Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology. 2014;155(4):1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su RW, Strug MR, Jeong JW, Miele L, Fazleabas AT. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci USA. 2016;113(8):2300–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015;100(3):E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357(1-2):108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. [DOI] [PubMed] [Google Scholar]
- 17. Schwanbeck R, Martini S, Bernoth K, Just U. The Notch signaling pathway: molecular basis of cell context dependency. Eur J Cell Biol. 2011;90(6-7):572–581. [DOI] [PubMed] [Google Scholar]
- 18. Kappou D, Matalliotakis M, Matalliotakis I. Medical treatments for endometriosis. Minerva Ginecol. 2010;62(5):415–432. [PubMed] [Google Scholar]
- 19. Vercellini P, Buggio L, Berlanda N, Barbara G, Somigliana E, Bosari S. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016;106(7):1552–1571.e2. [DOI] [PubMed] [Google Scholar]
- 20. Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34(7):1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haughian JM, Pinto MP, Harrell JC, Bliesner BS, Joensuu KM, Dye WW, Sartorius CA, Tan AC, Heikkilä P, Perou CM, Horwitz KB. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci USA. 2012;109(8):2742–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groeneweg JW, Hall TR, Zhang L, Kim M, Byron VF, Tambouret R, Sathayanrayanan S, Foster R, Rueda BR, Growdon WB. Inhibition of gamma-secretase activity impedes uterine serous carcinoma growth in a human xenograft model. Gynecol Oncol. 2014;133(3):607–615. [DOI] [PubMed] [Google Scholar]
- 23. Efferson CL, Winkelmann CT, Ware C, Sullivan T, Giampaoli S, Tammam J, Patel S, Mesiti G, Reilly JF, Gibson RE, Buser C, Yeatman T, Coppola D, Winter C, Clark EA, Draetta GF, Strack PR, Majumder PK. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 2010;70(6):2476–2484. [DOI] [PubMed] [Google Scholar]
- 24. Cui D, Dai J, Keller JMA, Mizokami A, Xia S, Keller ET. Notch pathway inhibition using PF-03084014, a ϒ-secretase inhibitor (GSI), enhances the antitumor effect of docetaxel in prostate cancer. Clin Cancer Res. 2015;21(20):4619–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296. [DOI] [PubMed] [Google Scholar]
- 26. American Society for Reproductive Medicine Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 27.RRID:AB_1977837.
- 28.RRID:AB_2164331.
- 29.RRID:AB_631470.
- 30.RRID:AB_310195.
- 31.RRID:AB_302459.
- 32. Tsai SJ, Wu MH, Lin CC, Sun HS, Chen HM. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86(12):5765–5773. [DOI] [PubMed] [Google Scholar]
- 33. Lin SC, Li YH, Wu MH, Chang YF, Lee DK, Tsai SY, Tsai MJ, Tsai SJ. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2014;99(3):E427–E437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RC. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97(3):E376–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang M, Wu L, Wang L, Xin X. Down-regulation of Notch1 by gamma-secretase inhibition contributes to cell growth inhibition and apoptosis in ovarian cancer cells A2780. Biochem Biophys Res Commun. 2010;393(1):144–149. [DOI] [PubMed] [Google Scholar]
- 36. Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, Ning L, Haymart M, Kunnimalaiyaan M, Chen H. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12(8):942–951. [DOI] [PubMed] [Google Scholar]
- 37. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang C, Zhang CJ, Martin BN, Bulek K, Kang Z, Zhao J, Bian G, Carman JA, Gao J, Dongre A, Xue H, Miller SD, Qian Y, Hambardzumyan D, Hamilton T, Ransohoff RM, Li X. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun. 2017;8:15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hsu EC, Kulp SK, Huang HL, Tu HJ, Salunke SB, Sullivan NJ, Sun D, Wicha MS, Shapiro CL, Chen CS. Function of integrin-linked kinase in modulating the stemness of IL-6-abundant breast cancer cells by regulating γ-secretase-mediated Notch1 activation in caveolae. Neoplasia. 2015;17(6):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grandi G, Mueller MD, Papadia A, Kocbek V, Bersinger NA, Petraglia F, Cagnacci A, McKinnon B. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J Reprod Immunol. 2016;117:30–38. [DOI] [PubMed] [Google Scholar]
- 41. Fortini F, Vieceli Dalla Sega F, Caliceti C, Aquila G, Pannella M, Pannuti A, Miele L, Ferrari R, Rizzo P. Estrogen receptor β-dependent Notch1 activation protects vascular endothelium against tumor necrosis factor α (TNFα)-induced apoptosis. J Biol Chem. 2017;292(44):18178–18191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153(6):2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnett-Mansfield RL, DeFazio A, Mote PA, Clarke CL. Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab. 2004;89(3):1429–1442. [DOI] [PubMed] [Google Scholar]
- 44. Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73(9-10):922–928. [DOI] [PubMed] [Google Scholar]
- 45. Li X-J, Liu X-J, Yang B, Fu YR, Zhao F, Shen ZZ, Miao LF, Rayner S, Chavanas S, Zhu H, Britt WJ, Tang Q, McVoy MA, Luo MH. Human cytomegalovirus infection dysregulates the localization and stability of NICD1 and Jag1 in neural progenitor cells. J Virol. 2015;89(13):6792–6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.