Figure 7.
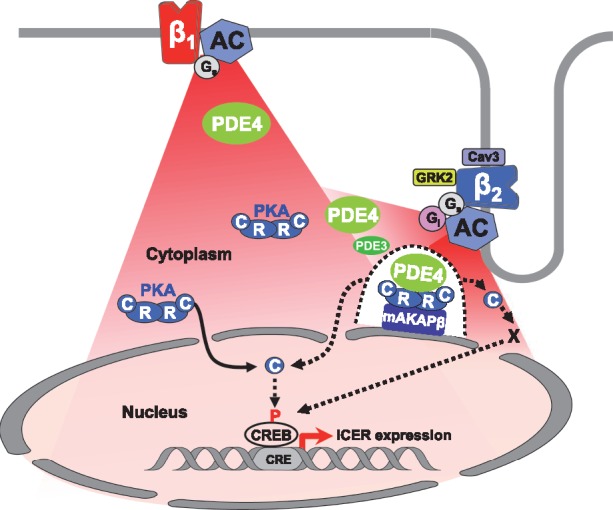
Proposed model for β1- and β2-AR regulation of cytoplasmic and nuclear PKA activity and ICER expression in adult cardiac myocytes. Stimulation of β1-ARs generate cAMP signals (in red) diffusing in the cytoplasm and the nucleus. Upon β1-ARs stimulation, PDE4 (in green) regulates cAMP levels to control PKA activity in the cytoplasm. A fraction of catalytic subunits (C) of PKA dissociate from regulatory subunits (R) and translocate inside the nucleus to increase nuclear PKA activity. Elevation of nuclear PKA activity allows induction of ICER transcription, presumably through CREB phosphorylation, which may be direct or indirect (dotted arrow between C and CREB). Stimulation of β2-ARs also generates cAMP elevation in the cytoplasm and the nucleus resulting in activation of cytoplasmic PKA. Cytoplasmic PKA activation upon β2-AR stimulation is restricted by caveolin3, Gi, GRK2, PDE3, and PDE4. In addition, PDE4 prevents activation by β2-ARs of a specific pool of PKA tethered by mAKAPβ at the perinuclear membrane (illustrated by the dotted line surrounding the mAKAPβ-PKA-PDE4 complex) which controls access of C subunits to the nucleus (dotted arrow) and nuclear PKA activation by β2-ARs. When PDE4 is inhibited, nuclear PKA activation contributes to ICER up-regulation by β2-ARs, although other mechanisms depending on cytoplasmic PKA activity are involved.
