To the Editor:
Statin-associated muscle symptoms (SAMS) are the most frequent side effect of statin therapy, but the absence of diagnostic markers for SAMS makes diagnosis and management of these symptoms difficult. This is increasingly important since clinicians and insurers seek to determine which patients are truly statin intolerant and warrant alternative cholesterol-lowering medications such as the newly approved and expensive PCSK9 inhibitors. Using creatine kinase (CK) as a diagnostic marker has been discouraged [1,2] because SAMS can occur in the absence of marked elevations of CK and CK is affected by factors such as race, gender, age, and recent exercise.
The Coenzyme Q10 in Statin Myopathy trial (NCT01140308) [3] enrolled men and women ≥20 yrs of age with a history of SAMS. Subjects were entered into a randomized, double-blind, crossover, lead-in trial of simvastatin (20 mg/d) or placebo for 8 weeks to confirm SAMS. Subjects then underwent a 4-week wash-out period, and were crossed over from simvastatin to placebo or vice versa. Muscle symptoms were documented weekly by telephone. CK as well as muscle pain (Pain Severity Score or PSS) [4] was measured before and after each treatment period. Only subjects who experienced muscle pain on simvastatin but not on placebo were classified as having confirmed SAMS. Informed consent was obtained from each patient according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Hartford Hospital Institutional Review Board.
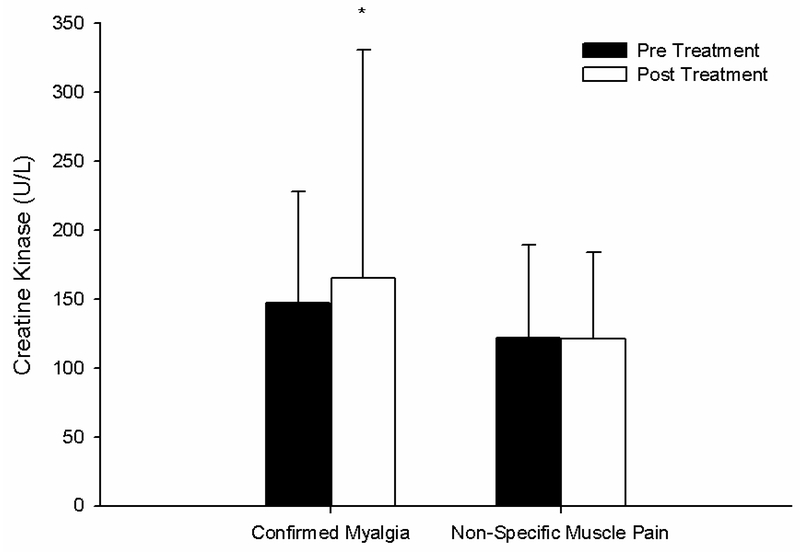
We reported that 43 patients experienced muscle pain on simvastatin alone (confirmed SAMS), with 35 experiencing pain on placebo alone, 21 experiencing pain on both treatments, and 21 experiencing no pain on either treatment [3]. For this short communication, we conducted a sub-analysis of the changes in CK and PSS among patients with confirmed SAMS versus the two groups of patients with pain on placebo alone and pain on both treatments. Among these 3 groups of patients, the change in CK with statin treatment was significantly higher (p = 0.04) in patients with confirmed SAMS (18 ± 150 U/L) vs. patients with pain on placebo alone or pain on both treatments (−1 ±49 U/L); Figure 1. The increase in pain severity with statin treatment was also significantly greater (p < 0.01) in patients with confirmed SAMS (from 0.3 ± 1.0 to 3.2 ± 2.2 PSS) vs. patients with pain on placebo alone or pain on both treatments (from 0.1 ± 0.4 to 0.2 ± 0.8 PSS). Moreover, the relationship between change in PSS and change in CK with statin therapy was influenced by SAMS status (p < 0.01) such that patients with confirmed SAMS exhibited a stronger positive relationship between change in CK and change in PSS (r2 = 0.036) than patients with non-specific muscle pain (r2 = 0.006).
Figure 1.
The group means ± standard deviation of creatine kinase before (Pre Treatment) and after (Post Treatment) simvastatin 20 mg in 43 patients who exhibited pain on simvastatin alone (confirmed myalgia) vs. patients who exhibited pain on both simvastatin and placebo or on placebo alone (non-specific muscle pain). * indicates significant change in creatine kinase from baseline within a group at p < 0.05.
These findings suggest that an increase in CK with statin treatment may be useful for distinguishing patients with true SAMS from patients with non-specific muscle pain. In our published report from this study [3], we also found that pre-treatment CK was higher in patients with confirmed SAMS than all other patients (152 ± 89 vs. 117 ± 68 U/L), and in a previous study we reported that 6 months of 80 mg/d atorvastatin treatment increased average CK by approximately 20 U/L [5], irrespective of SAMS status. These collective observations regarding subclinical elevations in CK with statin therapy are in contrast to most statin clinical trials, which have not typically reported CK on and off treatment except to report the number of patients experiencing CK > 10 times upper normal limits for safety purposes. Therefore, in this letter we present accumulating evidence that routinely measuring and reporting (especially in large clinical trials) pre- and post-treatment CK values may be valuable in treating, diagnosing, and better understanding the effects of statin therapy on skeletal muscle.
Acknowledgements
Work in this letter was funded by NIH/NCCAM RC1 AT005836.
Funder: NCCAM grant 1RC1AT005836 (P. Thompson).
Footnotes
Conflicts of Interest
Dr. Thompson has received research support from Genomas, Roche, Sanofi, Regeneron, Esperion, Amarin and Pfizer; has served as a consultant for Amgen, Regeneron, Merck, Genomas, Runners World, Sanofi, Esperion, and Amarin; has received speaker honoraria from Merck, Astra Zeneca, Kowa, and Amarin; owns stock in Abbvie, Abbott Labs, General Electric, J&J; and has provided expert legal testimony on exercise-related cardiac events and statin myopathy. Dr. Taylor served on the Pharmacovigilance Monitoring Board for Amgen, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stroes ES, Thompson PD, Corsini A, et al. , Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management, Eur. Heart J. 36 (2015) 1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA, An assessment by the Statin Muscle Safety Task Force: 2014 update, J. Clin. Lipidol. 8 (2014) S58–71. [DOI] [PubMed] [Google Scholar]
- [3].Parker BA, Gregory SM, Lorson L, Polk D, White CM, Thompson PD, A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design, J. Clin. Lipidol. 7 (2013) 187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tan G, Jensen MP, Thornby JI, Shanti BF, Validation of the Brief Pain Inventory for chronic nonmalignant pain, J. Pain 5 (2004) 133–7. [DOI] [PubMed] [Google Scholar]
- [5].Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, Chipkin S, Pescatello LS, Simpson K, White CM, Thompson PD, Effect of statins on skeletal muscle function, Circulation 127 (2013) 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]