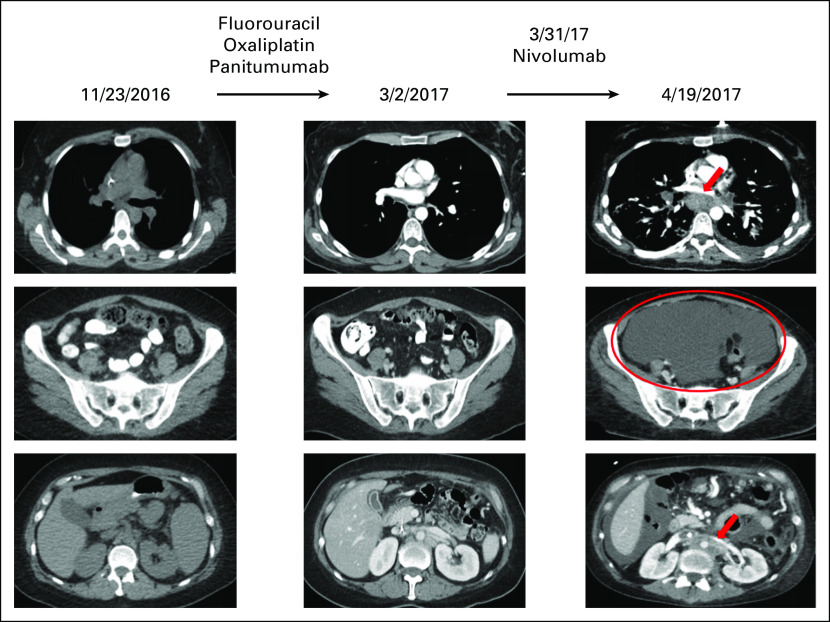
Fig 3.
Hyperprogression in a patient with MDM2 amplification treated with an anti–PD-1 checkpoint inhibitor.22 A 36-year-old woman presented with worsening dysphagia and anemia. Additional work-up revealed adenocarcinoma of the gastro-esophageal junction, stage IIIC. The patient was initially started on combination chemotherapy with epirubicin, oxaliplatin, and capecitabine with persistent lymphadenopathy. Therapy was switched to fluorouracil, oxaliplatin, and panitumumab with overall stable disease; however, the patient had persistent subcentimeter lymphadenopathy (left and middle). The regimen was then switched to nivolumab (anti–PD-1 inhibitor). Within 3 weeks, the patient showed marked clinical deterioration, and imaging showed rapid progression in mediastinal and retroperitoneal lymph nodes as well as emerging massive ascites (right). Pace of progression increased by 6.4-fold, tumor burden increased by 460% compared with pre-immunotherapy imaging, and time to treatment failure was 3 weeks (hyperprogression after immunotherapy previously defined as more than a two-fold increase in progression pace, a > 50% increase in tumor burden compared with pre-immunotherapy imaging, and a time to treatment failure < 2 months22). Therapy was then changed to fluorouracil, oxaliplatin, and trastuzumab, but the patient died 1.5 months after nivolumab was administered. Molecular profiling of the primary tumor revealed multiple alterations, including MDM2 amplification. Other alterations were ERBB3, ARAF, CDK4, and EGFR amplifications and alterations in PIK3CA, FRS2, GLI1, and IKZF1. Tumor mutation burden was low and microsatellite stable. PD-1 and PD-L1 status by immunohistochemistry were not evaluated.