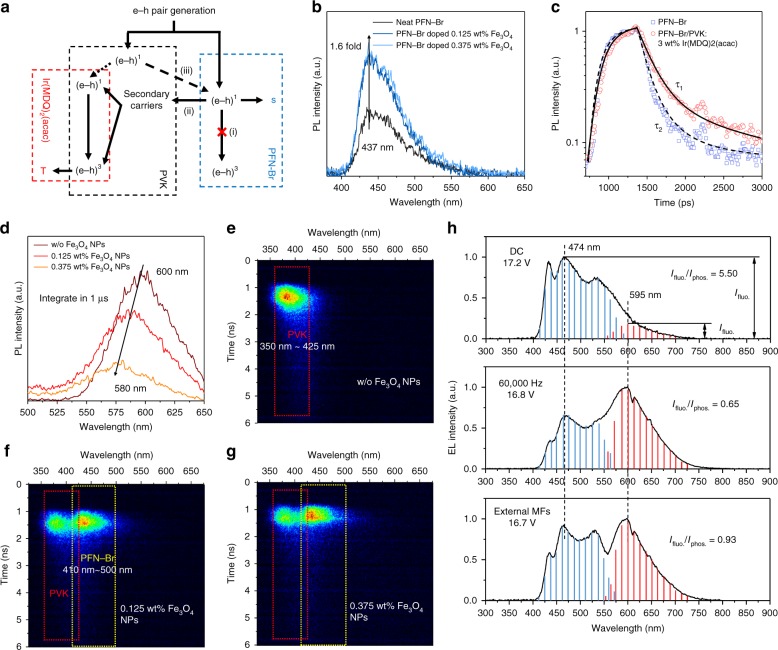
Fig. 5. Energy transfer analysis for the fluorescence–phosphorescence (F–P) emission unit in the presence of magnetic field effect.
a Schematic diagram of e–h pair excited energy transfer mechanisms among PVK (host), Ir(MDQ)2(acac) (phosphorescent dopant), and PFN-Br (fluorescent material) with magnetic-suppressive ISC. (e–h)1 and (e–h)3 represent singlet and triplet intermolecular e–h pairs. S and T are singlet and triplet excitons. b Time-integrated PL spectra that were measured at 437 nm for pure PFN-Br thin film, PFN-Br doping 0.125 wt% Fe3O4 NP film, and PFN-Br doping 0.375 wt% Fe3O4 NP film after constant excitation at 280 nm (10 Hz, 500 fs, and 130 μJ/cm2). c Time-resolved PL decay transients at 410–500 nm integration. d Time-integrated PL spectra (>1 μs) for glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br, glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br:0.125 wt% Fe3O4, and glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br:0.375 wt% Fe3O4. The streak images for glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br (e), glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br:0.125 wt% Fe3O4 (f), and glass/PVK:3 wt% Ir(MDQ)2(acac)/PFN-Br:0.375 wt% Fe3O4 (g) for 6 ns. h EL spectra with no magnetic field, internal AC magnetic field, and external magnetic field due to Fe3O4 NPs